Abstract
Entamoeba histolytica is an enteric tissue-invasive protozoan parasite causing amoebic colitis and liver abscesses in humans. Amoebic contact with host cells activates intracellular signaling pathways that lead to host cell death via generation of caspase-3, calpain, Ca2+ elevation, and reactive oxygen species (ROS). We previously reported that various NADPH oxidases (NOXs) are responsible for ROS-dependent death of various host cells induced by amoeba. In the present study, we investigated the specific NOX isoform involved in ROS-dependent death of hepatocytes induced by amoebas. Co-incubation of hepatoma HepG2 cells with live amoebic trophozoites resulted in remarkably increased DNA fragmentation compared to cells incubated with medium alone. HepG2 cells that adhered to amoebic trophozoites showed strong dichlorodihydrofluorescein diacetate (DCF-DA) fluorescence, suggesting intracellular ROS accumulation within host cells stimulated by amoebic trophozoites. Pretreatment of HepG2 cells with the general NOX inhibitor DPI or NOX2-specific inhibitor GSK 2795039 reduced Entamoeba-induced ROS generation. Similarly, Entamoeba-induced LDH release from HepG2 cells was effectively inhibited by pretreatment with DPI or GSK 2795039. In NOX2-silenced HepG2 cells, Entamoeba-induced LDH release was also significantly inhibited compared with controls. Taken together, the results support an important role of NOX2-derived ROS in hepatocyte death induced by E. histolytica.
-
Key words: Entamoeba histolytica, reactive oxygen species (ROS), NADPH oxidase (NOX), hepatocytes, cell death
Introduction
Entamoeba histolytica is a tissue-invasive extracellular protozoan parasite causing amoebic colitis and liver abscesses in humans [
1].
Entamoeba is known to cause inflammation of the large intestine and migrate through blood vessels to the liver, resulting in hepatitis and liver abscess [
1,
2]. It is estimated that
E. histolytica causes approximately 50,000,000 diarrheal infections and 100,000 deaths per year due to amoebic liver abscess [
2]. As little is known about the mechanism of liver cell destruction caused by
Entamoeba, amoebiasis remains an important disease worldwide. It is known that
Entamoeba enters the human body through contaminated water or food and, once in the intestine, secretes large amounts of cysteine proteases that degrade the mucin layer [
1,
2]. Contact with host cells via amoebic Gal-lectin induces host cell death [
1,
2]. In host cells, irreversible Ca
2+ increase, caspase 3 activation, calpain activation, and reactive oxygen species (ROS) production have been reported during the process of amoeba-induced cell death [
3–
6]. ROS function as secondary messenger molecules in various intracellular processes, including cell growth, death, and proliferation [
7–
9]. ROS generation by NADPH oxidase (NOX) is associated with host defense and cell death [
8]. Seven NOX isoforms have been identified: NOX1–5 and DUOX1,2 [
10]. All NOX isoforms have a dehydrogenase domain that binds a noncovalently linked flavin cofactor and NADPH substrate, with a common catalytic core comprising 6 transmembrane helices chelating 2 hemes [
9,
10]. Although the basic structure of the NOX isoforms is similar, their expression and distribution differ between tissues and cells [
9]. NOX2, the best-known NOX isoform, is primarily found in phagocytes and used as a defense mechanism to kill infecting bacteria via NOX2-derived ROS [
8]. Six homologs, namely NOX2, NOX1, NOX3, NOX4, NOX5, and DUOX1/2, are expressed in nonphagocytic cells and involved in various intracellular processes [
8,
9].
Entamoeba histolytica is known to induce ROS production in host cells, thereby leading to cell death [
11]. In a previous study, we reported that
Entamoeba activates the host cell NOX system to induce ROS generation. In neutrophils, which are phagocytic cells, NOX2-derived ROS production is induced by contact with live
Entamoeba [
6].
Entamoeba-induced ROS generation and cell death are reported to be suppressed when neutrophils are pretreated with the NOX inhibitor diphenyleneiodonium chloride (DPI) [
6]. Various NOX isoforms have been documented in amoeba-induced death of non-phagocytic cells. For example, ROS production by NOX1 is involved in the death of colon epithelial cells [
11,
17]. Furthermore, it was recently reported that Jurkat T cells express a high level of NOX4 and that NOX4-derived ROS produced by amoebae are closely related to Jurkat cell death [
18]. However, it remains unclear whether NOX-derived ROS are involved in
Entamoeba-induced human hepatocyte death. To address this research gap, the present study investigated the involvement of NOX-derived ROS in the mechanism of hepatocyte destruction caused by
Entamoeba histolytica.
Materials and Methods
Reagents
The NADPH oxidase DPI was purchased from Calbiochem (La Jolla, CA, USA). The NOX2-specific inhibitor GSK2795039 was purchased from MedChem Express (Monmouth Junction, NJ, USA). Mouse monoclonal antibodies against NOX2 were purchased from Santa Cruz Biotechnology (Delaware, CA, USA). Rabbit polyclonal antibodies against β-actin were purchased from Cell Signaling Technology (Beverly, MA, USA). Dichlorodihydrofluorescein diacetate (H2DCFDA) and 5-(and 6)-chloromethyl SNARF-1 were purchased from Molecular Probes (Eugene, OR, USA). Lipofectamine was purchased from Invitrogen (Carlsbad, CA, USA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
Cultivation of HepG2 cells and E. histolytica
HepG2 human hepatoma cells (American Type Culture Collection, Manassas, VA, USA) were grown in MEM media containing 10% (v/v) heat-inactivated FBS at 37°C in a humidified 5% CO2 atmosphere. Trophozoites of E. histolytica (HM1:IMSS) were grown axenically in TYI-S-33 medium and harvested after 48–72 h by chilling the culture tubes on ice for 10 min. After centrifugation at 200×g at 4°C for 5 min, trophozoites were washed with PBS and suspended in MEM containing 10% (v/v) heat-inactivated FBS. Cell viability, as assessed by the trypan blue exclusion assay, was consistently 99%.
Measurement of intracellular ROS generation in HepG2 cells
Intracellular ROS accumulation in HepG2 cells was assessed using the green fluorescent probe DCF-DA. HepG2 cells (1×104/well) were pre-stained at 37°C for 20 min with 5 μM DCF-DA, which rapidly oxidizes to highly fluorescent DCF in the presence of intracellular H2O2. ROS generation was analyzed using an inverted fluorescence microscope (Axiovert 200; Zeiss, Oberkochen, Germany). For spectrofluorometry, HepG2 cells were pretreated with an appropriate inhibitor or DMSO (v/v) before DCF-DA staining. Entamoeba cells (2×103/well) were pre-stained with DCF-DA for 10 min. HepG2 and Entamoeba cells were washed with PBS. Subsequently, HepG2 cells were incubated for up to 15 min with or without Entamoeba at a ratio of 5:1 (HepG2 cells to Entamoeba) in 96-well tissue culture plates in a CO2 incubator. Intracellular ROS production was using a Varioskan LUX (Thermo Scientific, Waltham, MA, USA) with excitation and emission wavelengths of 485 and 530 nm, respectively. Background fluorescence was subtracted using appropriate controls.
Measurement of E. histolytica-induced cell death using DNA fragmentation and LDH-release assays
To assess DNA fragmentation, HepG2 cells (4×106 cells/sample) were incubated with E. histolytica trophozoites (8×105 cells/sample) at a ratio of 5:1 (HepG2 cells to E. histolytica) for 60 min at 37°C in a humidified CO2 incubator. After incubation, cells were harvested by centrifugation and washed with cold PBS. DNA was extracted using a TaKaRa kit (MK600; Shiga, Japan) according to the manufacturer’s protocol. DNA was separated by 2% agarose gel electrophoresis and DNA fragmentation was visualized using ethidium bromide. LDH release was determined by evaluating the LDH content in the culture supernatant using a CytoTox 96 cytotoxicity assay system (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Briefly, 1 day before adding E. histolytica, HepG2 cells (2×105 cells/sample) were seeded on 24-well plates. Culture supernatant was collected after incubation with E. histolytica for 1 h at a 5:1 ratio (HepG2 cells to E. histolytica) and then centrifuged at 250×g for 4 min. The amount of LDH in the supernatant was measured using a 96-well microplate reader (Molecular Devices, Sunnyvale, CA, USA) according to the manufacturer’s protocol. The background value (spontaneous LDH release) was measured in non-stimulated cells and subtracted from each measurement value. Maximum LDH release was determined by incubating non-stimulated cells in lysis solution (1% Triton X-100 and PBS) at 37°C for 45 min.
NOX2 knockdown using siRNA in HepG2 cells
ON-target plus SMARTpool NOX2 siRNA (L-011021-00-0005) and control scrambled siRNA (D-001810-01-05) were purchased from Dharmacon (Lafayette, CO, USA). Cellular transfection of siRNA was performed using lipofectamine reagent according to the manufacturer’s instructions. At 72 h post-transfection, HepG2 cells were resuspended in fresh cell-culture medium for 1 h incubation with E. histolytica at a ratio of 5:1.
Western blot analysis
HepG2 cells (1×10
6 cells/sample) were incubated with or without
E. histolytica trophozoites (2×10
5 cells/sample). Next, the cells were pelleted by brief centrifugation and lysed on ice for 30 min using lysis buffer, as previously described [
11]. The samples were then subjected to SDS-PAGE and electrotransferred to Immobilon P PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% nonfat dry milk in TBST at room temperature for 1 h followed by overnight incubation with NOX2 or β-actin at 4°C. The membranes were then incubated with horseradish peroxidase-conjugated anti-rabbit antibody at room temperature for 1 h. Immunoreactivity was assessed using a LumiGLO system (Cell Signaling Technology, Danvers, MA, USA).
For each experiment, all reactions were performed in triplicate. The results are presented as the mean±SD of 3 to 5 independent experiments. Statistical analysis comparing results between experimental conditions was performed using Student’s t-test in Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA). Results with a P-value <0.05 were considered statistically significant.
Results
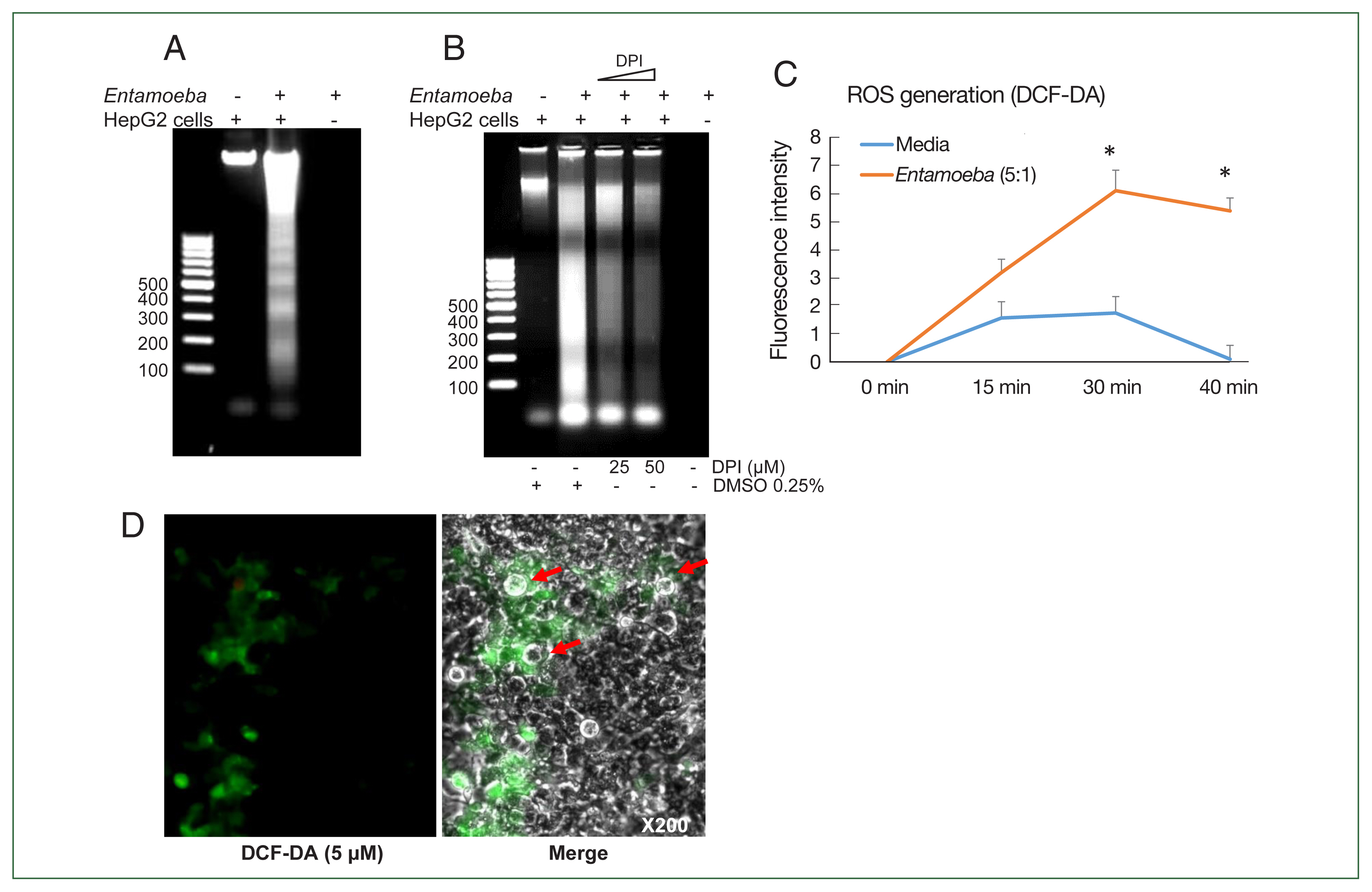
Co-incubation with E. histolytica induces DNA fragmentation and ROS generation in HepG2 cells
We first investigated whether co-incubation with
E. histolytica-induced DNA fragmentation in HepG2 cells. As shown in
Fig. 1A, HepG2 cells incubated with
Entamoeba for 1 h at a ratio of 5:1 (HepG2 cells to
E. histolytica) exhibited DNA fragmentation, whereas HepG2 cells incubated without
E. histolytica did not.
Entamoeba-induced DNA fragmentation in HepG2 cells treated with DPI was observed to decrease in a DPI concentration-dependent manner compared to the group without DPI (
Fig. 1B). To determine whether live amoebic trophozites induced ROS production in HepG2 cells, cells were pre-stained with the ROS indicator dye H2DCF-DA and
Entamoeba-triggered ROS production was then assessed by spectrofluorometry. ROS production in HepG2 cells co-incubated with trophozoites increased at 30 min, and was approximately threefold higher than that in the group without amoebae (
Fig. 1C). HepG2 cells treated with amoebae showed a strong green signal, indicating ROS production, compared to untreated cells (
Fig. 1D).
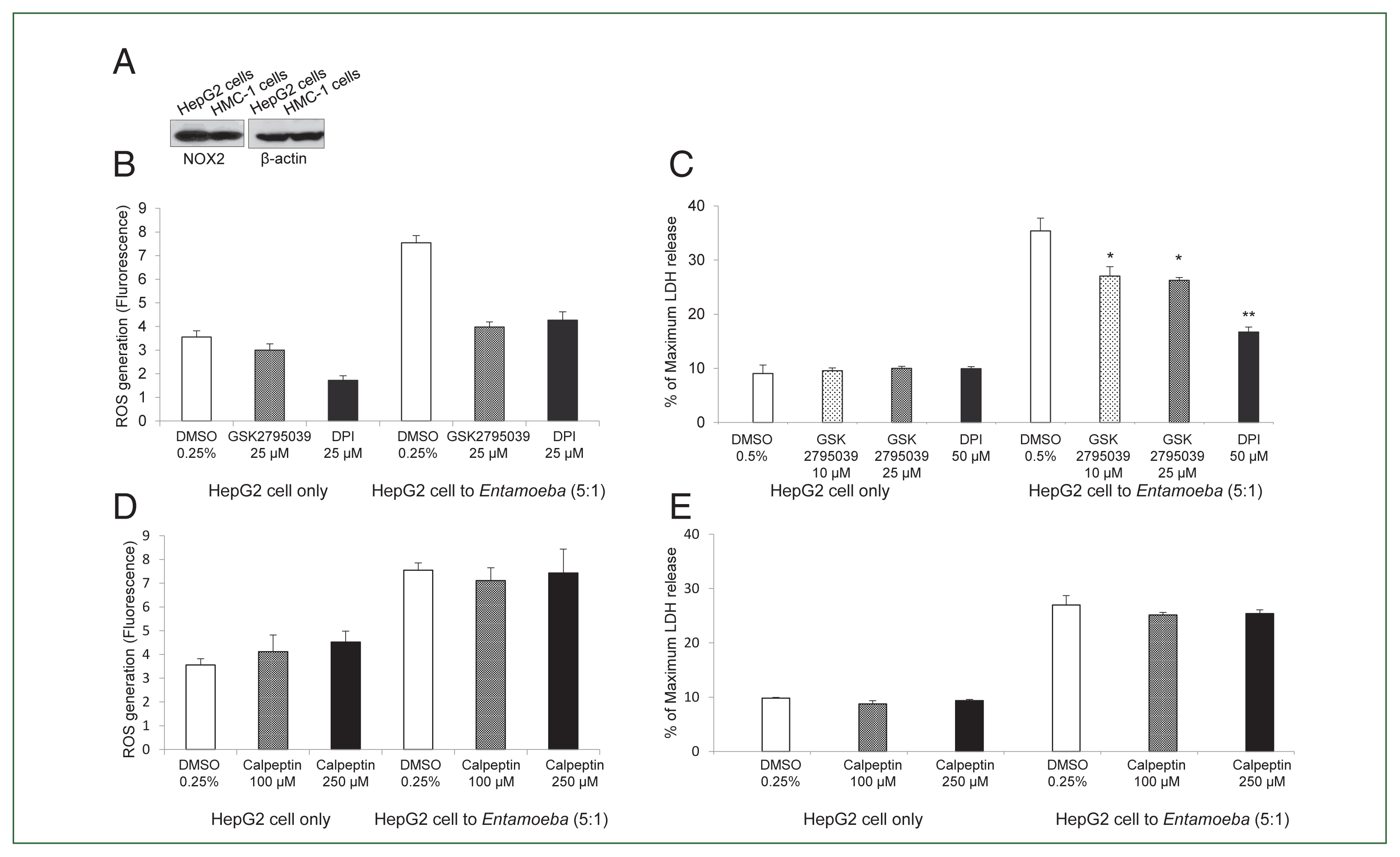
We then examined whether
Entamoeba-triggered ROS generation was induced by activation of the NOX system in HepG2 cells. HepG2 cells highly expressed NOX2 in the resting state, and NOX2 was also abundant in human mast cell line HMC-1 cells used as a control (
Fig. 2A). Co-incubation with live
Entamoeba strongly induced ROS production in HepG2 cells. However,
Entamoeba-induced ROS production in HepG2 cells was significantly reduced by pretreatment with the general NOX inhibitor DPI or specific NOX2 inhibitor GSK2795039 (
Fig. 2B).
Entamoeba-induced LDH release in HepG2 cells was also significantly reduced by pretreatment with the general NOX inhibitor DPI or specific NOX2 inhibitor GSK2795039 (
Fig. 2C).
Experiments testing the effect of calpain showed that
Entamoeba-induced ROS generation and LDH release in HepG2 cells were not inhibited by pretreatment with the calpain inhibitor calpeptin (
Fig. 2D, E).
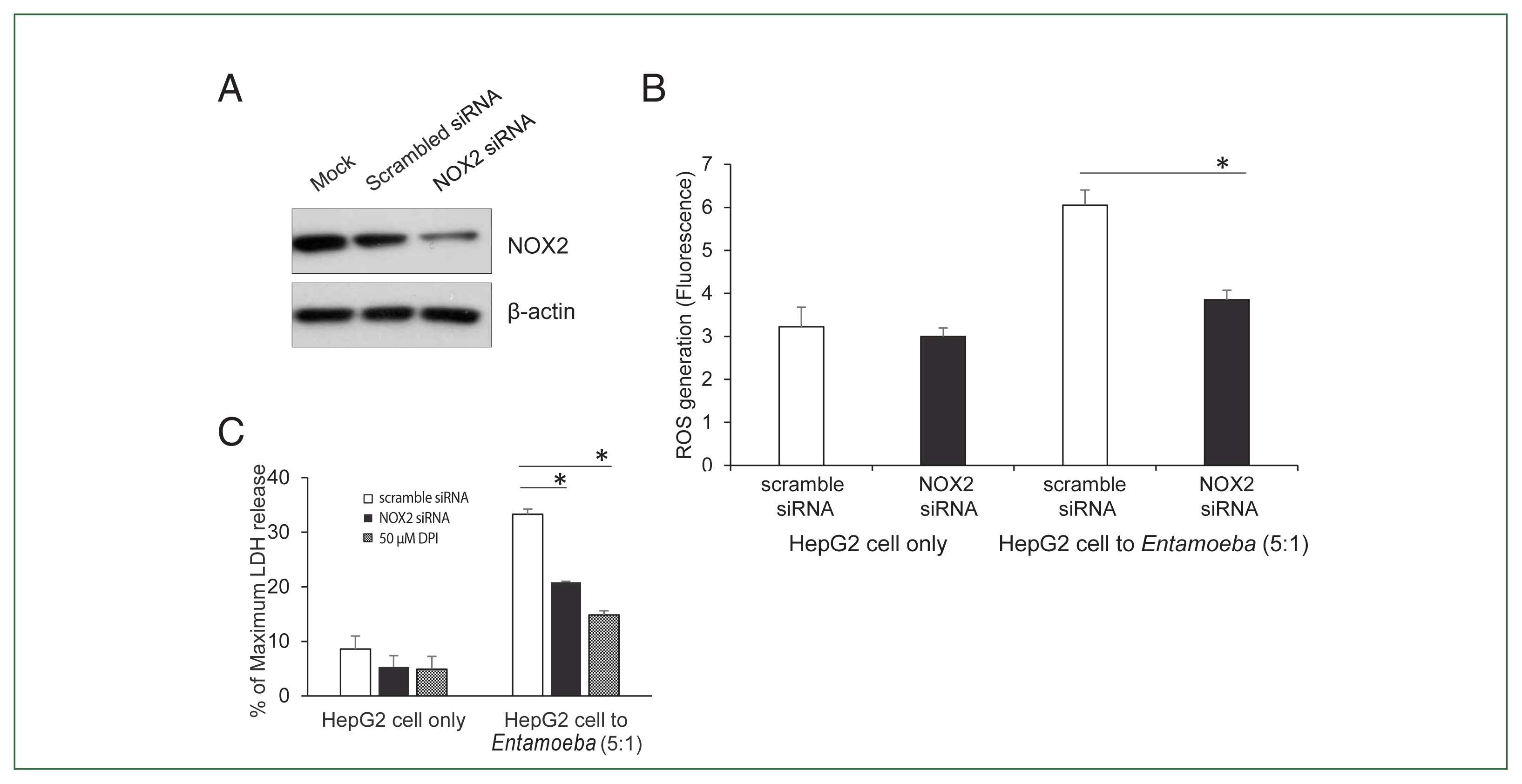
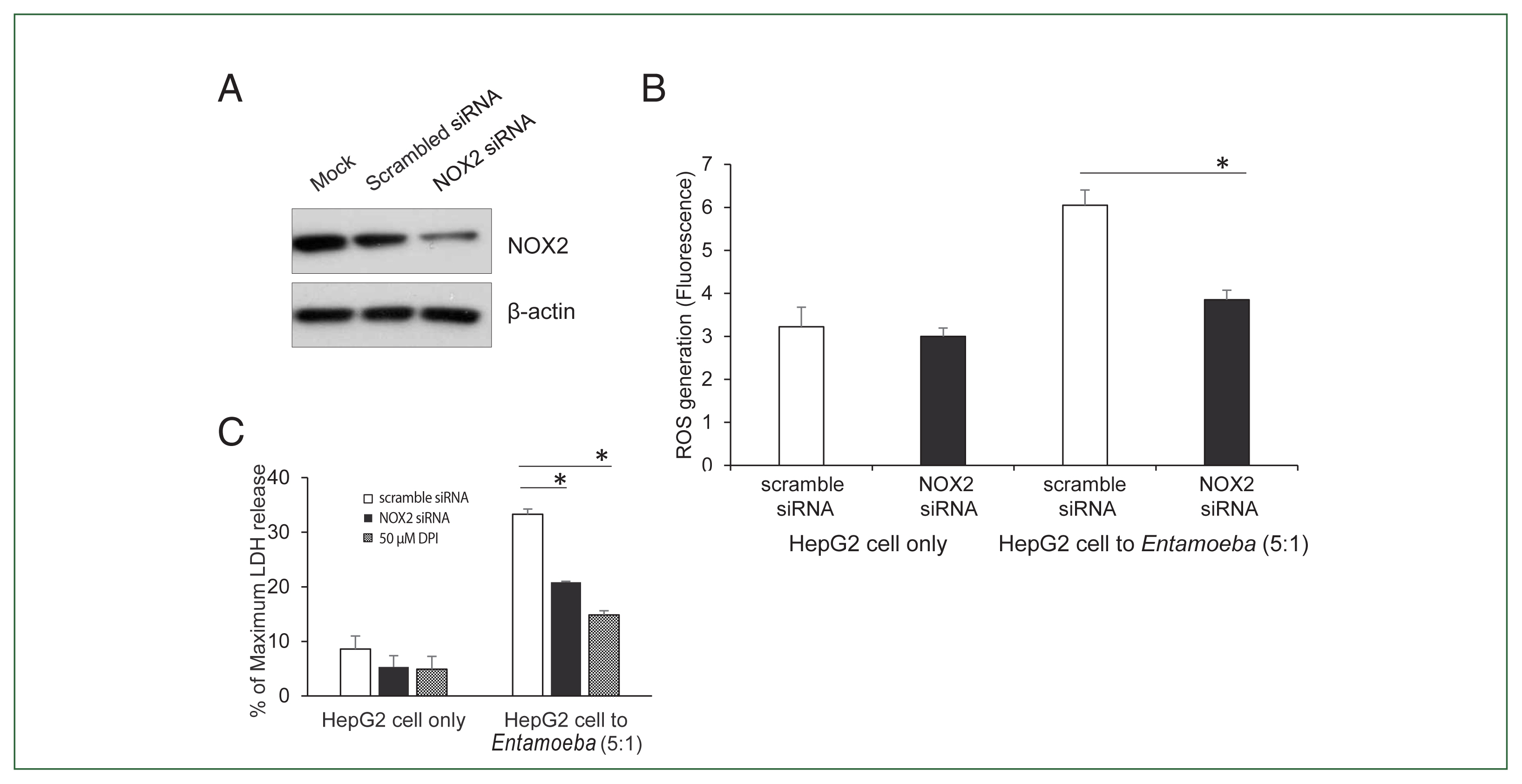
To verify the NOX2 isoform responsible for ROS generation in
Entamoeba-induced HepG2 cell death, HepG2 cells were transfected with the siRNA against NOX2. Knockdown of NOX2 protein was confirmed in HepG2 cells transfected with NOX2-siRNA (
Fig. 3A).
Entamoeba-induced ROS generation in HepG2 cells was measured by spectrofluorometry. As shown in
Fig. 3B,
Entamoeba-induced ROS generation in HepG2 cells transfected with NOX2 siRNA was significantly reduced compared to that in cells transfected with control siRNA. We then investigated the effect of NOX2-derived ROS on
Entamoeba-induced host cell death.
Entamoeba-induced LDH release in HepG2 cells pretreated with NOX2-siRNA was reduced by approximately 30% compared to the group treated with scrambled RNA (
Fig. 3C).
Discussion
Our study demonstrates the involvement of NOX2 in nonphagocytic cell death induced by E. histolytica for the first time. NOX2 was highly expressed in hepatoma HepG2 cells in the resting state and inhibition of NOX2 activity using the specific NOX2 inhibitor or NOX2 siRNA significantly reduced Entamoeba-induced ROS production and cell death in HepG2 cells. Taken together, these results suggest that NOX2-derived ROS play an important role in Entamoeba-induced death of non-phagocytic cells, such as hepatocytes.
It is generally accepted that NOX isoform expression is cell- or tissue-specific [
7]. In phagocytic cells (e.g., neutrophils, macrophages), NOX2 is predominant [
7,
9]. Other NOX isoforms (NOX1, NOX4, and NOX5) exist in non-phagocytic cells [
9]. NOX-derived ROS are closely related to cell death. For example, ionizing irradiation induces apoptotic damage in salivary gland acinar cells via NOX1-dependent superoxide generation [
12]. NOX2-derived ROS play an important role in
Mycobacterium infection-induced neutrophil apoptosis [
13]. NOX4 is involved in uric acid-induced death in normal human kidney HK-2 cells [
14]. NOX4-derived ROS is also reportedly involved in hepatocyte cell death during liver fibrosis [
15]. Various NOX-derived ROS are documented to influence host cell death induced by
E. histolytica [
6,
11,
16–
18]. For example, in phagocytotic cells like neutrophils, NOX2-derived ROS are essential for
Entamoeba-induced cell death [
6]. In colon epithelial cell lines such as Caco2 and HT-29 cells, which are non-phagocytic cells, NOX1-derived ROS plays an important role in cell death caused by
Entamoeba [
11,
17]. In a prior study, we reported that NOX4-derived ROS is involved in
Entamoeba-induced cell death in Jurkat T cells [
18]. These findings suggest that the specific NOX isoform involved in
Entamoeba-induced cell death differs depending on the host cell type.
NOXs, which are critical for host defense, are key producers of ROS in the liver [
19]. The human liver comprises several cell types, including hepatocytes, Kupffer cells, sinusoidal endothelial cells, and hepatic stellate cells [
19,
20]. Among these, hepatocytes are predominant and account for 70–85% of the cellular composition of the liver [
21]. Hepatocytes are responsible for various liver functions including detoxification, glycogen storage, metabolism, and plasma protein synthesis [
21]. NOX1 is relatively enriched in liver sinusoidal endothelial cells, while NOX2 is highly expressed in Kupffer cells [
19]. ROS generated by NOX2 in Kupffer cells play an important role in liver reperfusion injury. NOX1, NOX2, and NOX4 are also expressed in hepatocytes [
19]. Notably, NOX-derived ROS is closely related to hepatocyte cell death via necrosis or apoptosis. For example, NOX1 and NOX4 are involved in hepatocyte death during liver fibrosis [
15], and NOX4 is involved in HepG2 and HepG3B human hepatocyte cell death induced by TGFβ1 [
22]. NOX2 expression has been reported in human non-phagocytic cells such as cardiomyocytes, neurons, and lung epithelial cells. A prior study reported that NOX2 expression in human cardiomyocytes is increased after acute myocardial infarction [
23]. In neuronal cells infected with the Japanese encephalitis virus, NOX2-induced ROS production plays an important role in cell death [
24]. Furthermore, ROS generation via LPS-induced NOX2 expression in lung epithelial cells results in oxidative stress and inflammation [
13]. Activation of NOX2 in HepG2 cells was recently reported to be associated with nonalcoholic steatohepatitis during aging [
25]. Overall, these results are consistent with our finding that NOX2-derived ROS generation is required for cell death in HepG2 cells induced by
E. histolytica. However, in contrast to previous reports that activation of calpain is responsible for ROS production and cell death in Jurkat T cells and HT-29 colonic cells induced by
E. histolytica [
5,
26], the present study showed that calpain is not involved in ROS generation and death in HepG2 cells induced by
E. histolytica.
In conclusion, our results indicate that NOX2-derived ROS plays an important role in the death of hepatocytes induced by Entamoeba histolytica. Further research on the signaling mechanisms of cell death in various cell types in liver tissue induced by E. histolytica will provide a broader understanding of pathogenesis in liver abscess during human amoebiasis.
Notes
-
Author contributions
Conceptualization: Lee YA, Shin MH
Formal analysis: Lee YA
Funding acquisition: Shin MH
Investigation: Lee YA
Methodology: Lee YA
Project administration: Shin MH
Supervision: Shin MH
Visualization: Shin MH
Writing – original draft: Lee YA, Shin MH
Writing – review & editing: Lee YA, Shin MH
-
The authors declare no conflict of interest related to this study.
Acknowledgment
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST) (NRF-2023R1A2C1006708).
Fig. 1(A) E. histolytica-induced DNA fragmentation of HepG2 cells. HepG2 cells (4×106/sample) were incubated with or without E. histolytica (8×105/sample) for 60 min. (B) The effect of DPI on E. histolytica-induced DNA fragmentation in HepG2 cells. Cells (4×106/sample) pretreated with DPI (25 or 50 μM) or 0.5% (v/v) DMSO for 20 min were incubated in the absence or presence of E. histolytica (8×105/ sample) for 60 min. DNA fragmentation was analyzed by 2% agarose gel electrophoresis. The figure represents 3 separate experiments showing similar results. (C) E. histolytica-induced ROS generation in HepG2 cells determined by microfluorometery. HepG2 cells (5×104/sample) pretreated with 5 μM H2DCF-DA for 30 min at 37°C in a CO2 incubator were incubated with or without E. histolytica (1×104/sample), after which production of intracellular ROS was determined. Data are presented as the mean±SD of 3 independent experiments. *P values<0.05 were considered statistically significant. (D) E. histolytica-induced ROS generation in HepG2 cells observed by fluorescent microscopy. HepG2 cells pretreated with 5 μM H2DCF-DA for 30 min at 37°C in a CO2 incubator were co-incubated with amoebae. E. histolytica are marked by red arrows. ×200.
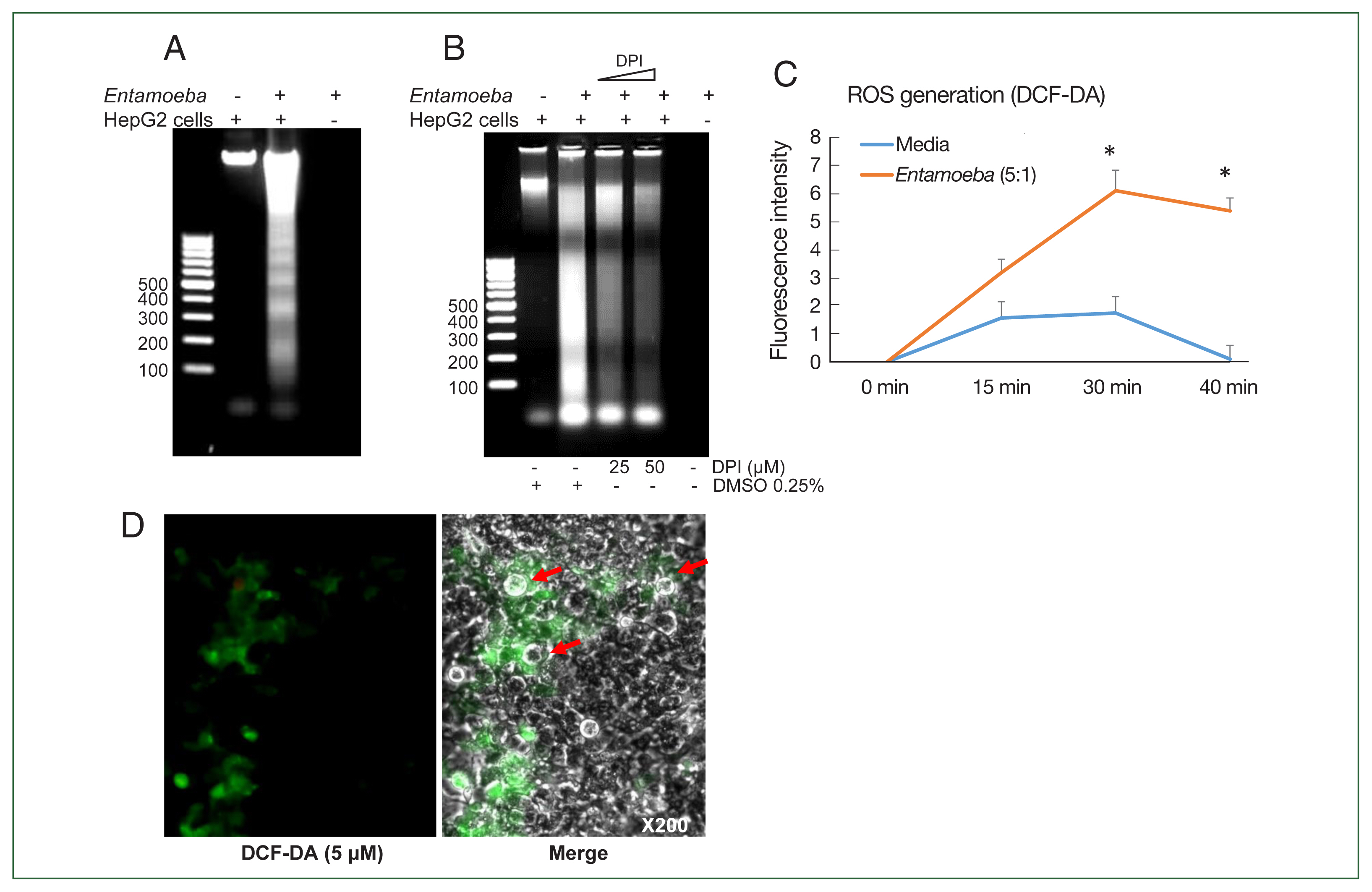
Fig. 2(A) NOX2 expression in HepG2 cells in the resting state. Whole cell lysates were subjected to SDS-PAGE and blotted with an anti-NOX2 antibody. The image represents 3 experiments showing similar results. (B) Effect of the NOX inhibitor DPI or NOX2-specific inhibitor GSK2795039 on Entamoeba-induced ROS generation in HepG2 cells. E. histolytica-induced ROS generation in HepG2 cells determined by microfluorometery. Data are presented as the mean±SD of 3 independent experiments. (C) Effect of NOX2 inhibitor on Entamoeba-induced HepG2 cell death. LDH release in the supernatant was assayed. Relative cytotoxicity (%) was calculated based on the released LDH. The figure represents 3 experiments showing similar results. *P<0.05, **P<0.01. (D) Effect of calpeptin on Entamoeba-induced ROS generation. E. histolytica-induced ROS generation in HepG2 cells assessed by microfluorometery. Intracellular ROS production was determined at excitation and emission wavelengths of 485 and 530 nm. Data are presented as the mean±SD of 3 independent experiments. (E) Effect of calpeptin on Entamoeba-induced HepG2 cell death. HepG2 cells (1×105/sample) pretreated with calpeptin (100 or 250 μM) or 0.25% DMSO (v/v) as a control for 15 min were incubated with E. histolytica (2×104/sample) at a 5: 1 cell ratio (HepG2 cells to E. histolytica) for 1 h at 37°C in a CO2 incubator, after which LDH released in the supernatant was assayed. Relative cytotoxicity (%) was calculated based on the released LDH. The image represents 3 experiments showing similar results.
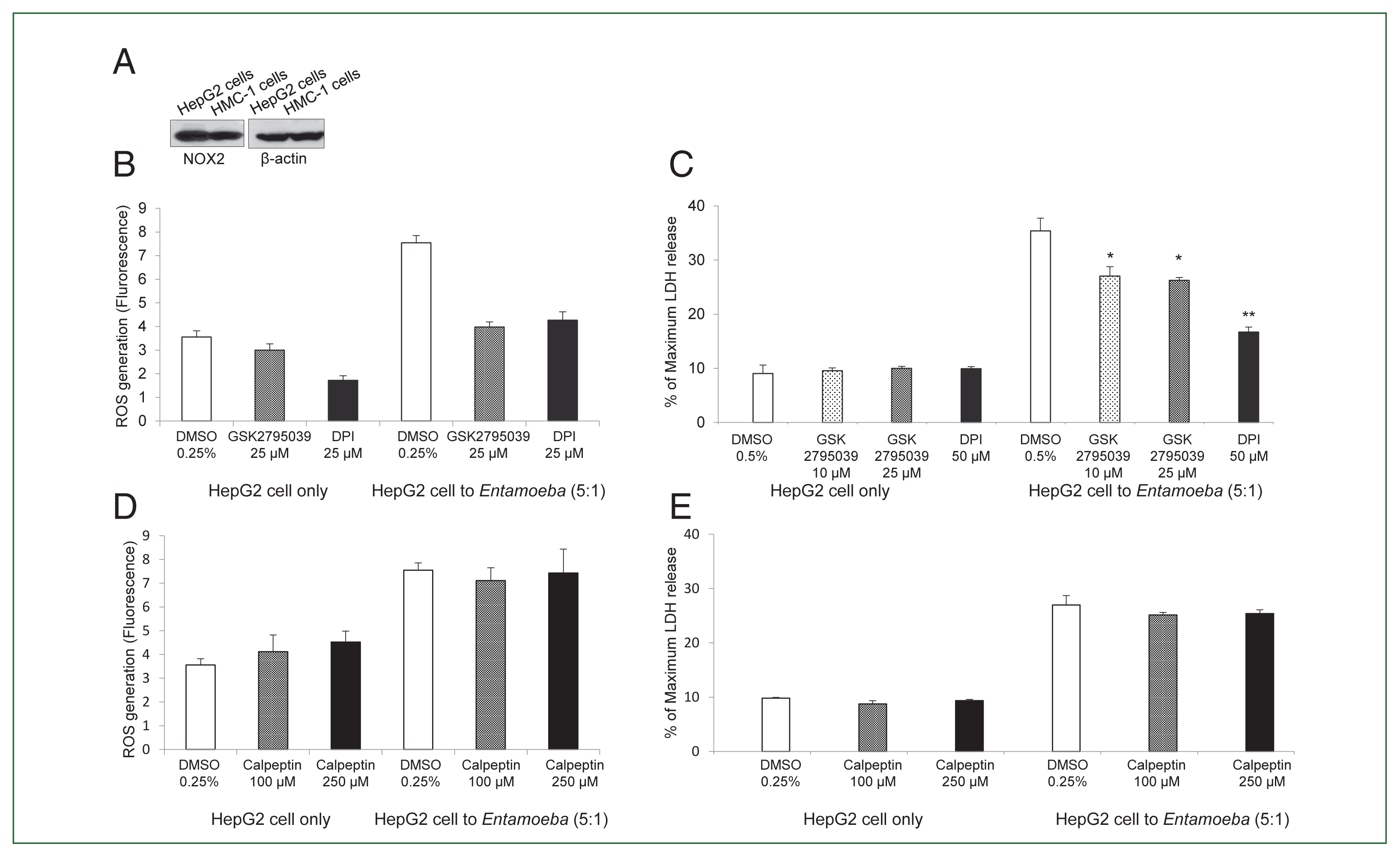
Fig. 3(A) NOX2 protein levels in HepG2 cells determined by immunoblotting after NOX2 gene silencing using siRNA. At 72 h post-transfection, whole-cell lysates from HepG2 transfected with empty vehicle (Mock), control scrambled siRNA (100 nM), or NOX1 siRNA (100 nM) were subjected to immunoblotting with anti-NOX2. Anti-β-actin antibody was used as the loading control. Blots represent 3 independent experiments. (B) Effect of siRNA for NOX2 on Entamoeba-induced ROS generation. At 72 h post-transfection, HepG2 cells (1×105), control scrambled siRNA (100 nM), or NOX1 siRNA (100 nM), were co-incubated for 15 min with amoebae. Intracellular ROS production was determined by spectrofluorometry. Data are presented as the mean±SEM of 3 independent experiments. Significant differences between groups are indicated as follows: *P<0.05. (C) Effect of siRNA against NOX2 on Entamoeba-induced HepG2 cell death. HepG2 cells (1×105/sample) transfected with siRNA against NOX2 or scramble RNA were incubated with E. histolytica (2×104/sample) at a 5:1 cell ratio (HepG2 cells to E. histolytica) for 1 h at 37°C in a CO2 incubator, after which LDH release was assayed. Relative cytotoxicity (%) was calculated based on the released LDH. The figure represents 3 experiments showing similar results. *P<0.05.
References
- 1. Stanley SL JR, Reed SL. Microbes and microbial toxin: paradigms for microbial-mucosal interactions. VI. Entamoeba histolytica: parasite-host interactions. Sm J Physiol Gastrointest Liver Physiol 2001;280(6):1049-1054.
https://doi.org/10.1152/ajpgi.2001.280.6.G1049
- 2. Ralston KS. Taking a bite: amoebic trogocytosis in Entamoeba histolytica and beyond. Curr Opin Microbiol 2015;28:26-35.
https://doi.org/10.1016/j.mib.2015.07.009
- 3. Ravdin JI, Moreau F, Sullivan JA, Petri WA Jr, Mandell GL. Relationship of free intracellular calcium to the cytolytic activity of Entamoeba histolytica
. Infect Immun 1988;56(6):1505-1512.
https://doi.org/10.1128/iai.56.6.1505-1512.1988
- 4. Huston CD, Houpt ER, Mann BJ, Hahn CS, Petri WA Jr. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica.
. Cell Microbiol 2000;2(6):617-625.
https://doi.org/10.1046/j.1462-5822.2000.00085.x
- 5. Kim KA, Lee YA, Shin MH. Calpain-dependent calpastatin cleavage regulates caspase-3 activation during apoptosis of Jurkat T cells induced by Entamoeba histolytica.
. Int J Parasitol 2007;37(11):1209-1219.
https://doi.org/10.1016/j.ijpara.2007.03.011
- 6. Sim S, Yong TS, Park SJ, Im KI, Kong Y, et al. NADPH oxidase-derived reactive oxygen species-mediated activation of ERK1/2 is required for apoptosis of human neutrophils induced by Entamoeba histolytica.
. J Immunol 2005;174(7):4279-4288.
https://doi.org/10.4049/jimmunol.174.7.4279
- 7. Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res 2006;71(2):289-299.
https://doi.org/10.1016/j.cardiores.2006.05.004
- 8. Breitenbach M, Rinnerthaler M, Weber M, Breitenbach-Koller H, Karl T, et al. The defense and signaling role of NADPH oxidases in eukaryotic cells. Wien Med Wochenschr 2018;168(11–12):286-299.
https://doi.org/10.1007/s10354-018-0640-4
- 9. Guichard C, Pedruzzi E, Fay M, Ben Mkaddem S, Coant N, et al. The Nox/Duox family of ROS-generating NADPH oxidases. Med Sci (Paris) 2006;22(11):953-959. (in French). https://doi.org/10.1051/medsci/20062211953
- 10. Petropolis DB, Faust DM, Jhingan GD, Guillen N. A new human 3D-liver model unravels the role of galectins in liver infection by the parasite Entamoeba histolytica.
. PLoS Pathog 2014;10(9):e1004381.
https://doi.org/10.1371/journal.ppat.1004381
- 11. Kim KA, Kim JY, Lee YA, Song KJ, Min D, et al. NOX1 participates in ROS-dependent cell death of colon epithelial Caco2 cells induced by Entamoeba histolytica.
. Microbes Infect 2011;13(12–13):1052-1061.
https://doi.org/10.1016/j.micinf.2011.06.001
- 12. Tateishi Y, Sasabe E, Ueta E, Tetsuya . Yamamoto Ionizing irradiation induces apoptotic damage of salivary gland acinar cells via NADPH oxidase 1-dependent superoxide generation. Biochem Biophys Res Commun 2008;366(2):301-307.
https://doi.org/10.1016/j.bbrc.2007.11.039
- 13. Sul OJ, Ra SW. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules 2021;26(22):6949.
https://doi.org/10.3390/molecules26226949
- 14. Li Z, Sheng Y, Liu C, Li K, Huang X, et al. Nox4 has a crucial role in uric acid-induced oxidative stress and apoptosis in renal tubular cells. Mol Med Rep 2016;13(5):4343-4348.
https://doi.org/10.3892/mmr.2016.5083
- 15. Sancho P, Mainez J, Crosas-Molist E, Roncero C, Fernández-Rodriguez CM, et al. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One 2012;7(9):e45285.
https://doi.org/10.1371/journal.pone.0045285
- 16. Lee YA, Sim S, Kim KA, Shin MH. Signaling Role of NADPH oxidases in ROS-Dependent host cell death induced by pathogenic Entamoeba histolytica.
. Korean J Parasitol 2022;60(3):155-161.
https://doi.org/10.3347/kjp.2022.60.3.155
- 17. Kim KA, Kim JY, Lee YA, Min A, Bahk YY, et al.
Entamoeba histolytica induces cell death of HT29 colonic epithelial cells via NOX1-derived ROS. Korean J Parasitol 2013;51(1):61-68.
https://doi.org/10.3347/kjp.2013.51.1.61
- 18. Lee YA, Kim KA, Min A, Shin MH. NOX4 activation is involved in ROS-dependent Jurkat T-cell death induced by Entamoeba histolytica.
. Parasite Immunol 2019;41(11):e12670.
https://doi.org/10.1111/pim.12670
- 19. De Minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys 2007;462(2):266-272.
https://doi.org/10.1016/j.abb.2007.04.016
- 20. Liu J, Huang X, Werner M, Broering R, Yang D, et al. Advanced method for isolation of mouse hepatocytes, liver sinusoidal endothelial cells, and kupffer cells. Methods Mol Biol 2017;1540:249-258.
https://doi.org/10.1007/978-1-4939-6700-1_21
- 21. Tsilimigras DI, Brodt P, Clavien PA, Muschel RJ, D’Angelica MI, et al. Liver metastases. Nat Rev Dis Primers 2021;7(1):27.
https://doi.org/10.1038/s41572-021-00261-6
- 22. Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, et al. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol 2008;49(6):965-976.
https://doi.org/10.1016/j.jhep.2008.07.021
- 23. Krijnen PA, Meischl C, Hack CE, Meijer CJ, Visser CA, et al. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J Clin Pathol 2003;56(3):194-199.
https://doi.org/10.1136/jcp.56.3.194
- 24. Singh G, Kumar A. Japanese encephalitis virus infection causes an imbalance in the activation of mitochondrial fusion/fission genes and triggers the activation of NOX2-mediated oxidative stress and neuronal cell death. Neurochem Res 2023;48(7):2196-2205.
https://doi.org/10.1007/s11064-023-03898-9
- 25. Jiang JX, Fish SR, Tomilov A, Li Y, Fan W, et al. Nonphagocytic activation of NOX2 is implicated in progressive nonalcoholic steatohepatitis during aging. Hepatology 2020;72(4):1204-1218.
https://doi.org/10.1002/hep.31118
- 26. Jang YS, Song KJ, Kim JY, Lee YA, Kim KA, et al. Calpains are involved in Entamoeba histolytica-induced death of HT-29 colonic epithelial cells. Korean J Parasitol 2011;49(2):177-180.
https://doi.org/10.3347/kjp.2011.49.2.177