Abstract
Tick infestation causes a significant threat to human and animal health, requiring effective immunological control methods. This study aimed to investigate the potential of recombinant Haemaphysalis longicornis enolase protein for tick vaccine development. The exact mechanism of the recently identified enolase protein from the H. longicornis Jeju strain remains poorly understood. Enolase plays a crucial role in glycolysis, the metabolic process that converts glucose into energy, and is essential for the motility, adhesion, invasion, growth, and differentiation of ticks. In this study, mice were immunized with recombinant enolase, and polyclonal antibodies were generated. Western blot analysis confirmed the specific recognition of enolase by the antiserum. The effects of immunization on tick feeding and attachment were assessed. Adult ticks attached to the recombinant enolase-immunized mice demonstrated longer attachment time, increased blood-sucking abilities, and lower engorgement weight than the controls. The nymphs and larvae had a reduced attachment rate and low engorgement rate compared to the controls. Mice immunized with recombinant enolase expressed in Escherichia coli displayed 90% efficacy in preventing tick infestation. The glycolytic nature of enolase and its involvement in crucial physiological processes makes it an attractive target for disrupting tick survival and disease transmission. Polyclonal antibodies recognize enolase and significantly reduce attachment rates, tick feeding, and engorgement. Our findings indicate that recombinant enolase may be a valuable vaccine candidate for H. longicornis infection in experimental murine model.
-
Key words: Tick, immunity, antigen, biological control, recombinant enolase
Introduction
Tick infestations and their diseases pose a global threat to human and animal health. The spread of tick-borne diseases is expanding, and identifying effective strategies to control this spread is critical. The hard tick
Haemaphysalis longicornis is widely distributed in East Asia and Australia and can cause infections leading to degenerative disorders in both humans and animals [
1,
2]. Therefore, developing new strategies to combat tick infestations and tick-borne diseases is crucial.
Some hosts become resistant after multiple tick infestations and develop immune responses to substances contained in tick saliva [
3–
5]. Consequently, alternative methods for the control of ticks are urgently required. The threat to human and animal health posed by tick infestations and the diseases they carry is a growing concern worldwide. While chemical acaricides are often ineffective against ticks, tick vaccines are effective and sustainable for controlling tick infestations and tick-borne diseases. However, there is a pressing need for other effective strategies to control these pests. Overall, the global threat of tick-borne illnesses requires continued attention and action from public health officials and the scientific community.
Vaccination with tick extracts is thought to result in immunological protection that can make people resistant to infection [
3,
4,
6]. The effectiveness of such techniques depends on the identification and cloning of tick molecules involved in the mediation of key physiological roles [
7]. Tick vaccines are an attractive method for controlling ticks, but their efficacy needs to be improved. Recently, the development of vaccines against
Boophilus spp. has provided novel opportunities to identify protective antigens suitable for integration into vaccines that can be used to control tick infestations effectively [
8]. Ticks are the most significant carriers of infections causing disease in both domestic and wild animals; they are ranked second to mosquitoes as disease vectors for humans worldwide [
9]. Molecular processes at the tick-pathogen interface enable pathogens to infect and grow in both ticks and vertebrate hosts [
9].
Consequently, tick control is of medical and veterinary relevance. It may be accomplished through various techniques, with synthetic acaricides becoming the backbone for tick control in animals in recent decades. However, acaricide-resistant tick species have started to appear, necessitating alternate tick management strategies [
10].
Purified BMA7 and Bm86 from
Rhipicephalus microplus were used to co-vaccinate calves, which resulted in significant anti-Bm86 (4200–94000) and anti-BmA7 (1600–22000) antibody titers and a reduction in the feeding tick egg masses [
8]. Cattle challenge infestations were decreased by 97% when treated with Bm86 from yeast and Subolesin from
Escherichia coli [
11]. Furthermore, challenge infestations were decreased by 97% in cattle treated with a combination of
E. coli and yeast-expressed Subolesin and Bm86 antigens [
12].
In Brazil, TickGardPLUS (Intervet Australia) and GavacTM (Heber Biotec S.A., Cuba) exhibited lower effectiveness rates (49.2% and 46.4%, respectively) compared to vaccines from other countries [
13]. The optimal vaccine efficacy should be greater than 50%, as demonstrated in tests employing recombinant proteins identified by sialotranscriptome analysis, where the vaccine had an effectiveness of 73.2% [
14]. Two vaccinations containing recombinant Bm86 were later licensed for use in Australia (TickGARD) and South American countries (Gavac) between 1993 and 1997 [
15]. Host resistance to infestation can be determined by measuring tick feeding and fecundity performance parameters; several of these parameters have been shown to be affected by hosts resistant to other tick species [
16,
17]. The enolase protein inhibits 2 important metabolic pathways vital for cellular function: the reversible conversion of D-2-phosphoglycerate (2PGA) and phosphoenolpyruvate (PEP) in glycolysis and gluconeogenesis and the production of ATP; however, it may interfere with the attachment and invasion process of the parasite [
18].
This study aimed to determine the possibility of using enolase as a candidate vaccine antigen for tick control. Our findings show that enolase may be a viable candidate antigen for the production of H. longicornis subunit vaccines with appropriate immunogenicity and protective efficacy.
Materials and Methods
Ethics statement
All animals used in these experiments were housed in the Laboratory of Veterinary Parasitology, College of Veterinary Medicine and Bio-Safety Research Institute, Jeonbuk National University (Jeonju, Korea). All animal studies and protocols were compliant with the ethical principles of animal research and approved by the Jeonbuk Animal Care and Use Committee (JBNU 2022–094).
Ticks and mice
The Jeju strain of the hard tick H. longicornis has been cultured in our laboratory since 2003; the ticks are fed mouse blood and kept in an incubator at a temperature of 25°C and humidity level of 95%.
Expression of recombinant enolase in E. coli
The H. longicornis enolase gene (GenBank accession number ON871822) was inserted into the EcoRI site of the pBluescript SK (+) vector. After digestion with EcoRI, the gene was subcloned into the pGEMEX-2 E. coli expression vector (Promega, Madison, WI, USA). The accuracy of the insertion of the resulting plasmid, pGEMEX-2/ENOLASE, was checked via restriction enzyme analyses. pGEMEX-2/ENOLASE was used to transform Escherichia coli (JM109 [DE3], Promega) using standard techniques. The expressed recombinant enolase (rHlEno) was produced as a fusion protein with gene 10 (the gene 10-enolase protein).
Mouse immunization
Eighteen 8-week-old female BALB/c mice were immunized and challenged with H. longicornis ticks. The mice were divided into 2 groups (n=9 each): One received immunization with rHlEno, and the other received the gene 10 protein (control). Each mouse was intraperitoneally injected with 100 μg of either rHlEno or gene 10 protein emulsified with Freund’s complete adjuvant. The first and second booster injections, each with Freund’s incomplete adjuvant, were administered at 2-week intervals.
Serological analysis
Host immune responses to the recombinant proteins were determined by immunoblot analysis, as described previously [
19]. Nitrocellulose membranes were probed for rHlEno and gene 10 proteins, and antibody titers were expressed as the highest dilution showing immune reactive bands.
When the antibody titers reached 1:5,000–1:8,000, the rabbits were challenged with
H. longicornis at different developmental stages. Unfed larvae, nymphs, and adult
H. longicornis were allowed to feed on the shaved ears of the rHlEno- or gene 10-immunized rabbits, as described previously [
1]. The infestations involved 3 adult ticks, 10 nymphs, and 30 larvae per mouse. Following tick infestation, the mice were visually examined, during which the following parameters were recorded: pre-feeding periods, feeding periods, attachment rates, engorged weights, molting periods, and egg weights. The engorged ticks were obtained, transferred to individual glass flasks, and placed in an incubator set at 25°C with a relative humidity of approximately 85% to allow for egg-laying and molting. The time between the start of infestation and attachment was defined as the pre-feeding period. Feeding periods were defined as the duration between the attachment and completion of engorgement. The attachment rates were determined based on the rates from infestation to engorgement. The engorged weights were measured immediately after detachment, and the egg weights were measured 3 weeks later.
The values are reported as mean±standard error, and group mean comparisons were conducted using Student’s t-test. Differences in means were considered significant at the 95% confidence level. Statistical tests were performed using GraphPad Prism V6.01 software (GraphPad Software, Inc.), and a P-value of <0.05 was considered significant.
Results
Recombinant enolase was produced using the pGEMEX-2 vector (10-enolase) within the
E. coli host system. The recombinant enolase protein (10-enolase) was genetically fused with gene 10, and subsequent analysis through SDS-PAGE gel electrophoresis (
Supplementary Fig. S1) revealed that the expressed recombinant protein (rHlEno) had an approximate molecular weight of 83-kDa after isopropyl β-D-1-thiogalactopyranoside (IPTG) induction. To further confirm the presence of the recombinant 10-enolase protein, Western blot analysis was conducted using anti-mouse enolase immune sera, which positively identified the protein of interest.
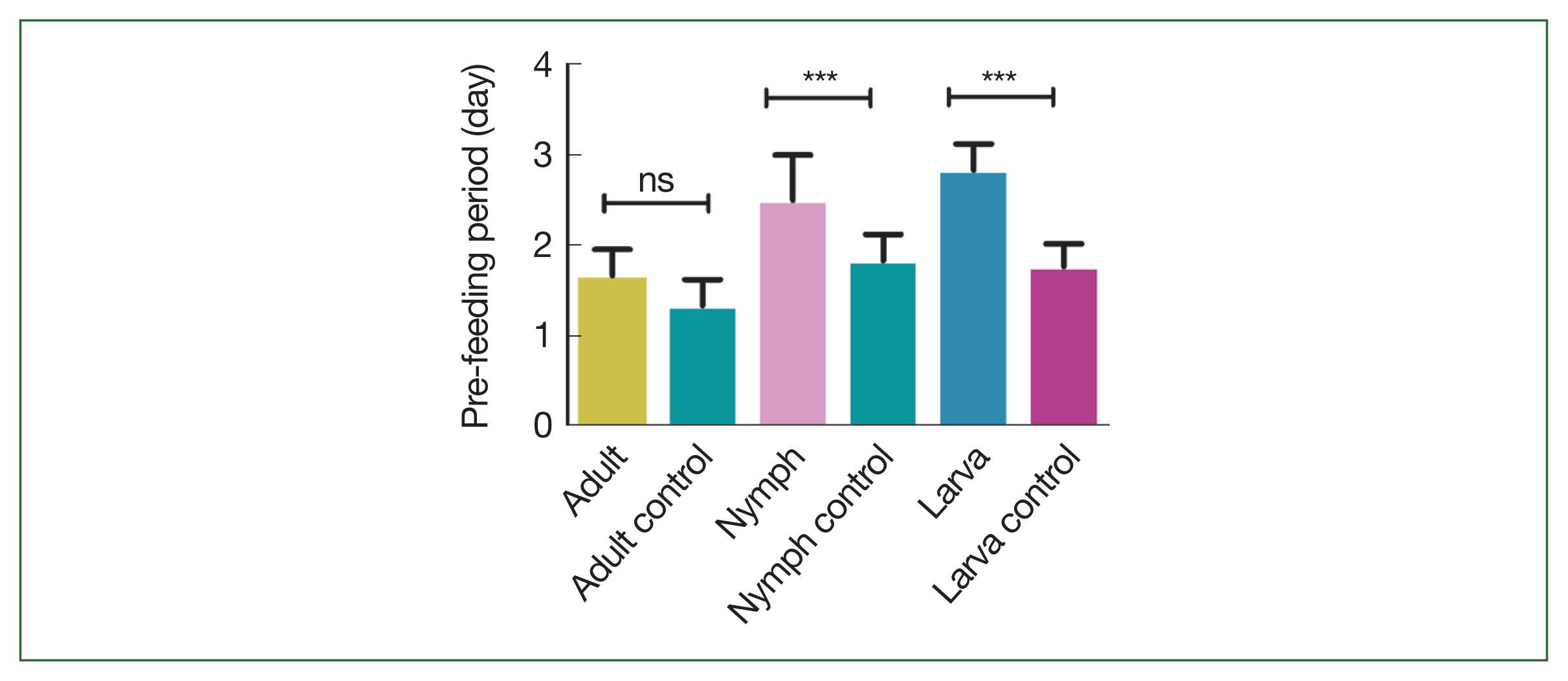
The effects of rHlEno immunization on tick feeding in mice are scrutinized. No differences in attachment rates were observed during the first 24 h after nymphal and adult ticks were introduced onto the backs of the mice. Longer pre-feeding periods were observed in adult ticks that fed on rHlEno-immunized mice during the larval, nymphal, and adult stages; the time of infestation to attachment was 2±0.17 days compared with 1±0.17 days in the controls (
P<0.05;
Fig. 1). Significance differences in pre-feeding periods were observed between the nymphs (3±0.29 days) and larvae (3±0.17 days) compared to those in the controls (1.5±0.17 and 1.8±0.15 days, respectively;
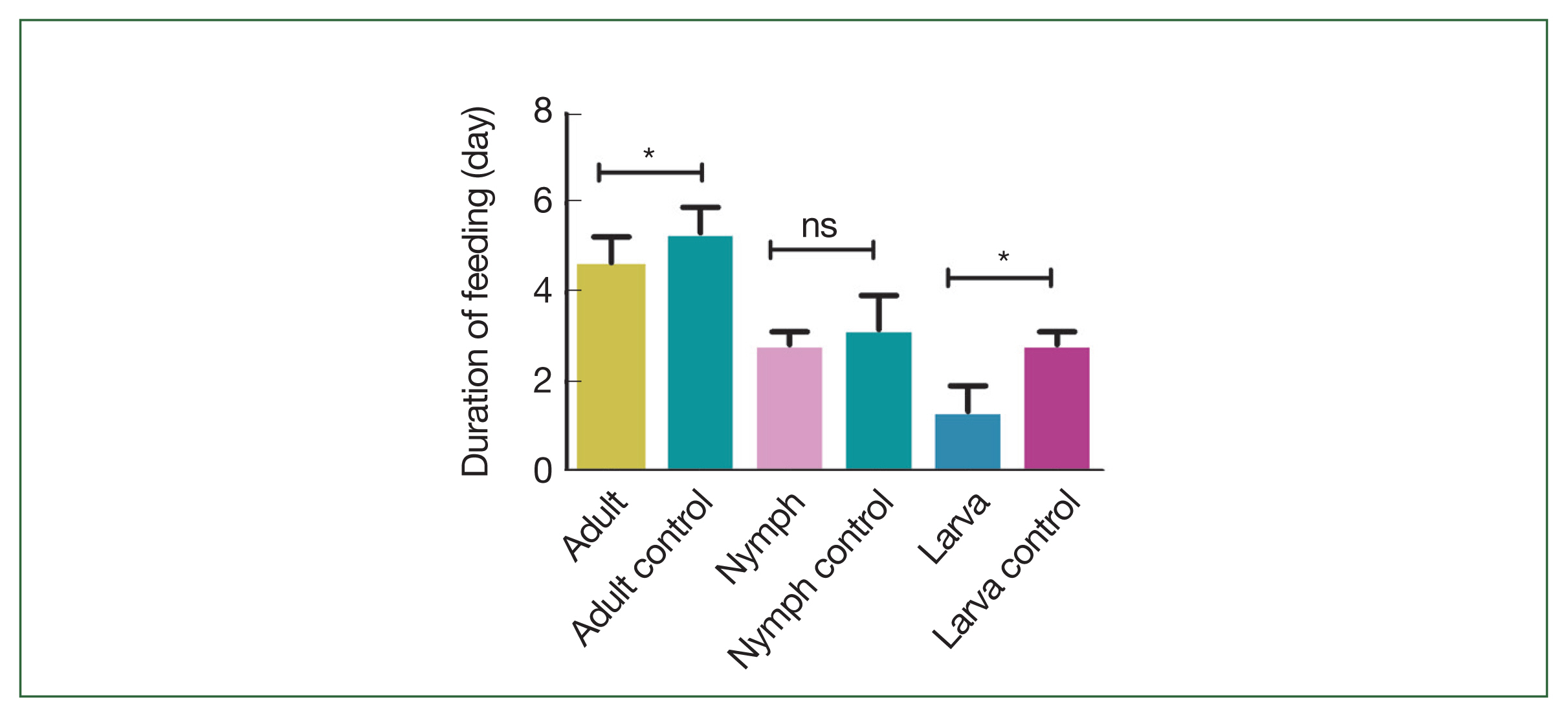
Fig. 1). Both larval (1.3±0.6 days) and adult (4.7±0.6 days) ticks that fed on rHlEno-immunized mice significantly longer feeding periods (
P<0.05) compared to the controls (2.8±0.6 days and 5.3±0.6 days, respectively;
Fig. 2). The nymphal tick feeding period in the rHlEno-immunized mice (2.8±0.3 days) was lower than that in the controls (3.2±0.8 days).
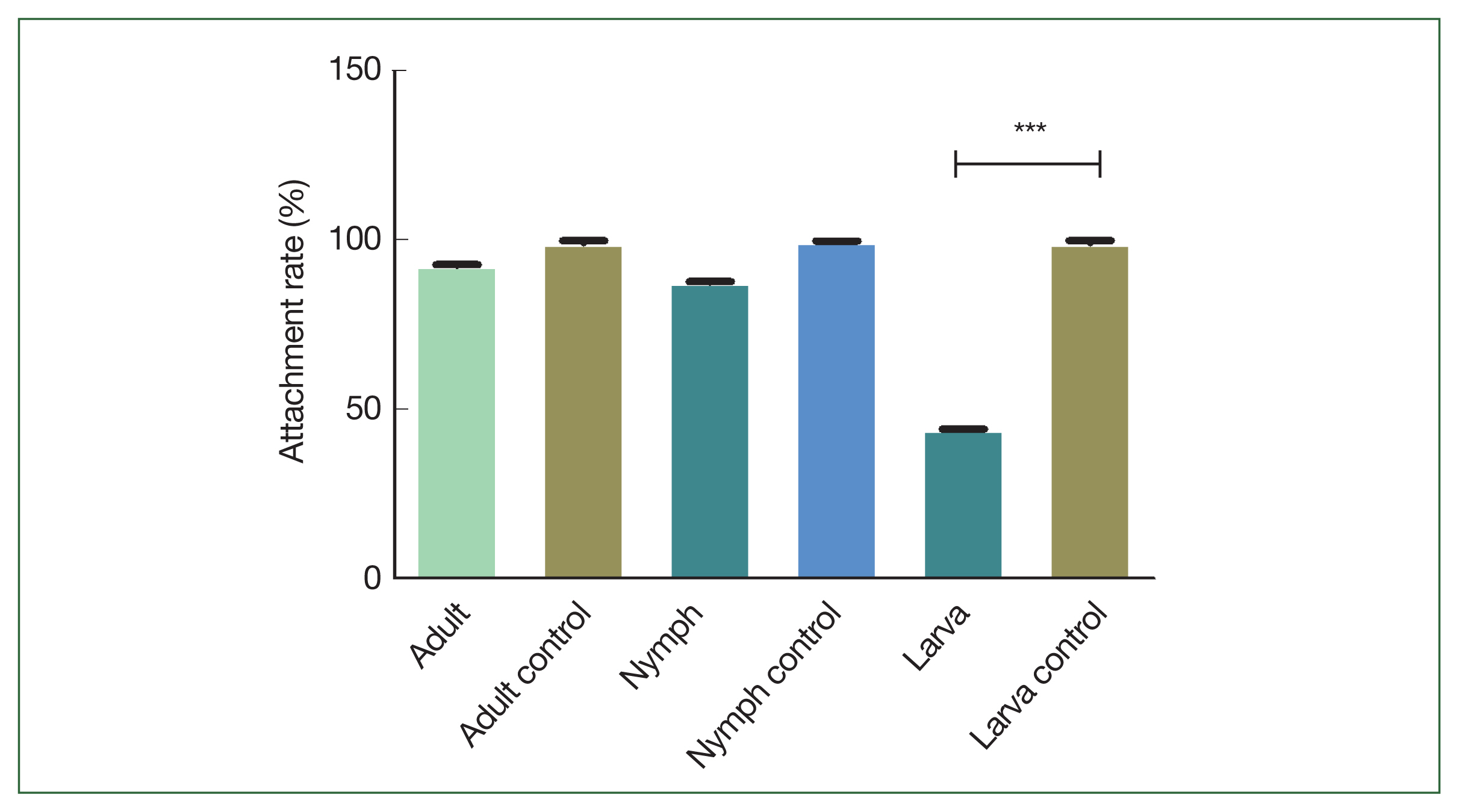
As shown in
Fig. 3, larval ticks that fed on rHlEno-immunized mice exhibited a significantly lower attachment rate (44.4%) compared to the controls (100%); no significant differences were observed during the nymph (88.9%) and adult (93.3%) stages.
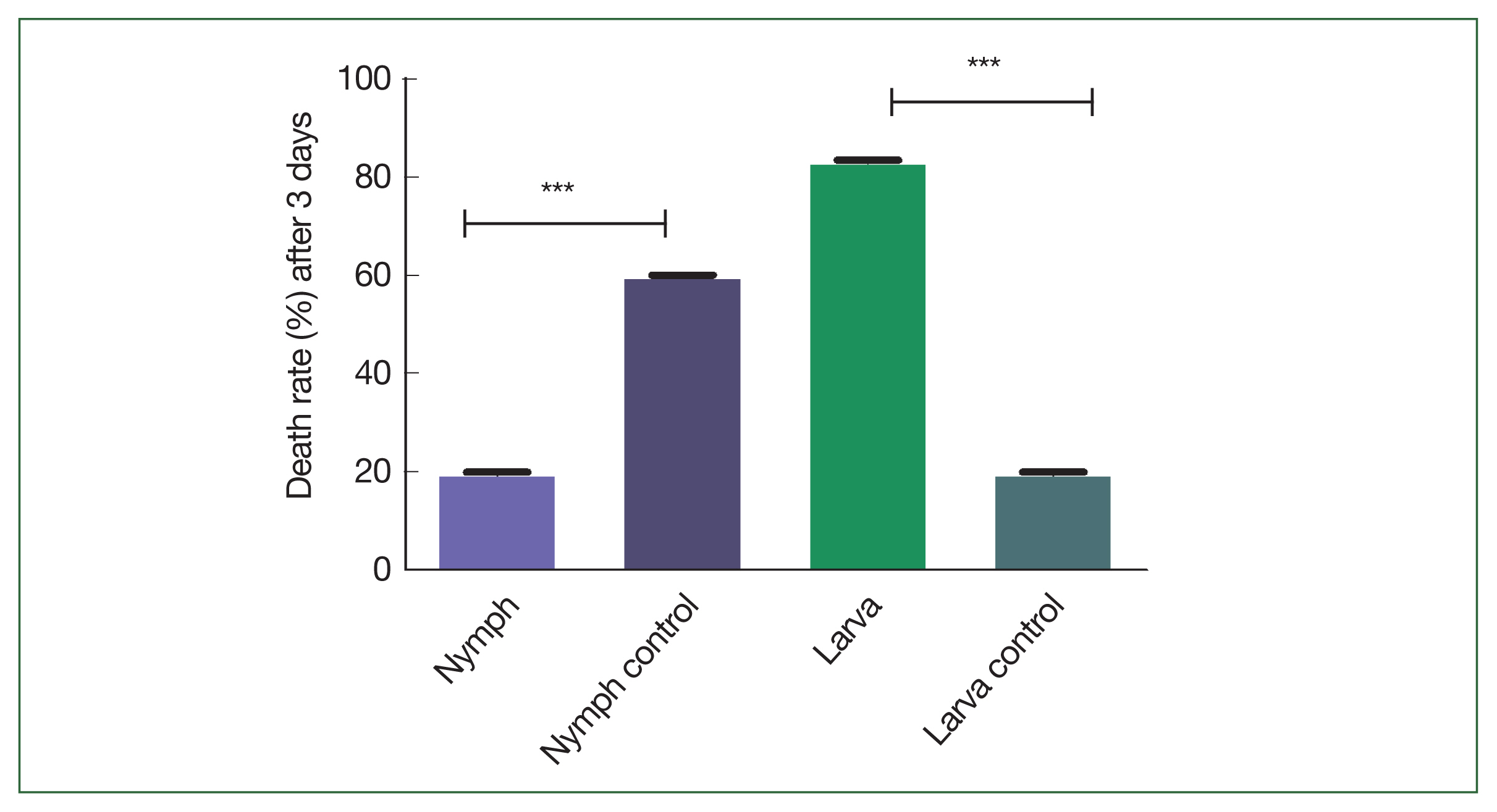
Adult ticks in rHlEno-immunized mice could not feed on blood properly compared to the control adult ticks. Approximately 60% of nymphs and 83.33% of larvae in the rHlEno-immunized group died compared to those in the control group (20%;
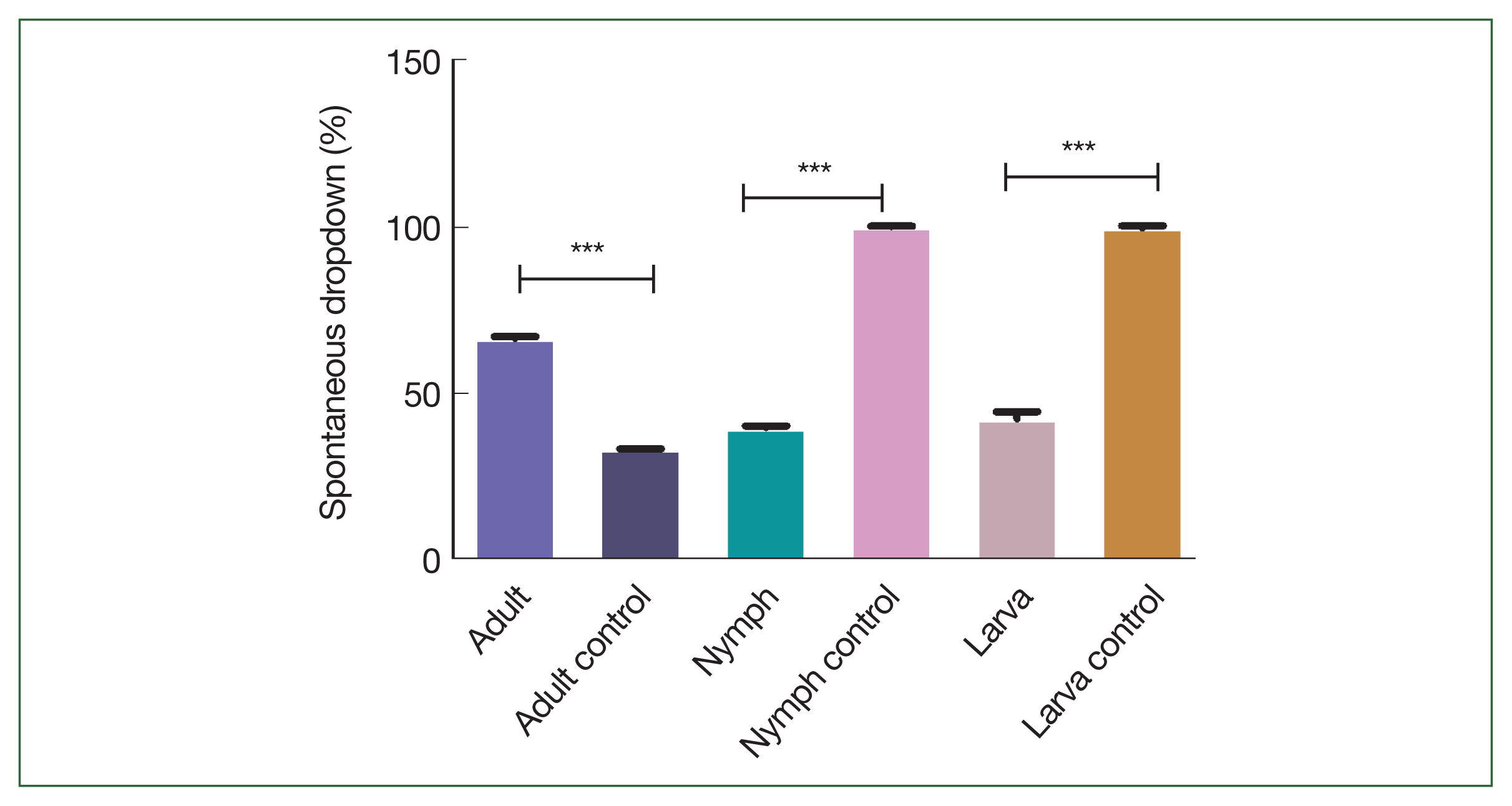
Fig. 4). The spontaneous dropping percentages of the adult, nymph, and larva ticks after 3 days of feeding in the immunized mice (66.7%, 100%, and 100%, respectively) were significantly higher than those in the controls (33.3%, 40%, and 40%, respectively;
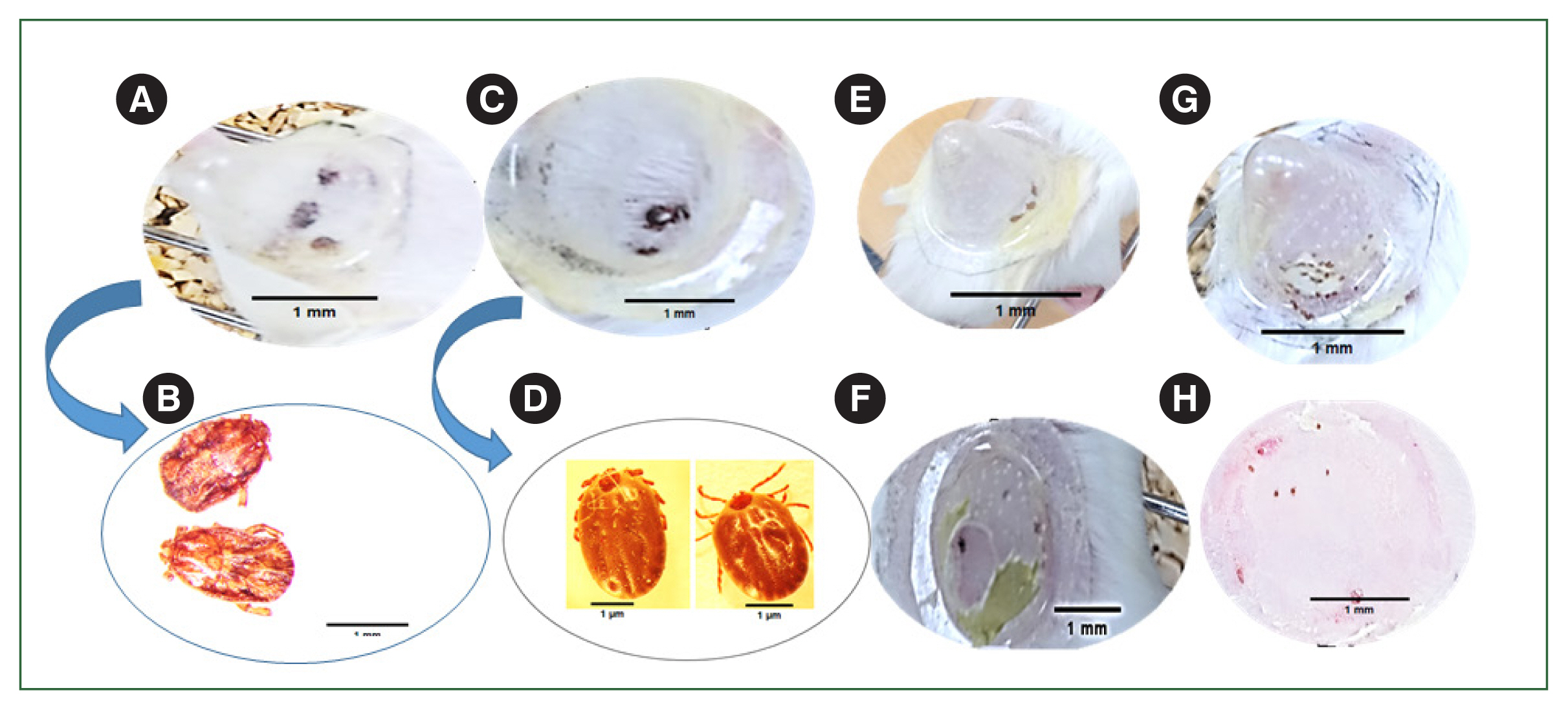
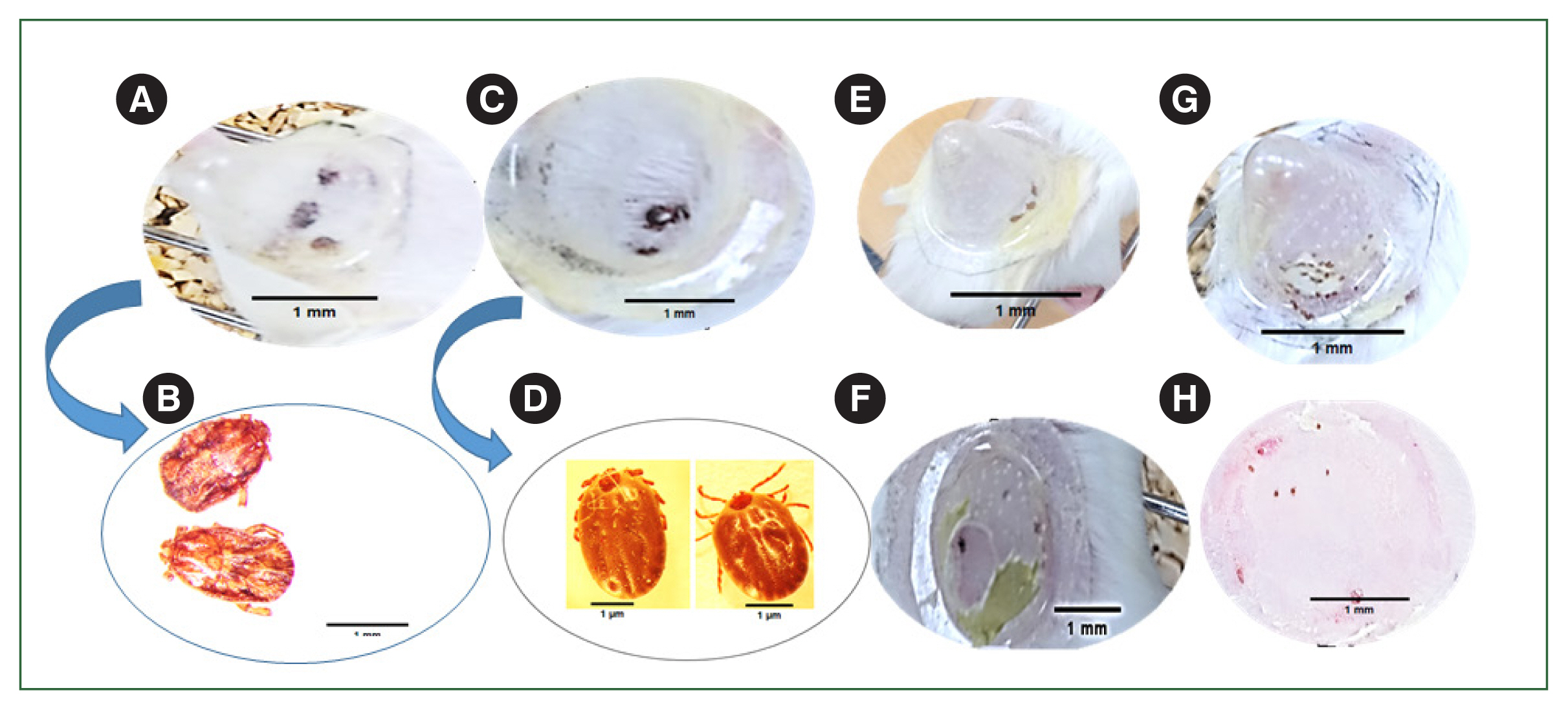
Fig. 5). Significant phenotypic changes were observed following infestation with the ticks during the adult (
Fig. 6A, B), nymph (
Fig. 6E), and larval (
Fig. 6G), stages in the rHlEno-immunized mice compared to those in the gene 10 protein-immunized control mice showed Adult (
Fig. 6C, D), nymph (
Fig. 6F), and larva (
Fig. 6H).
Discussion
The global livestock sector is facing an escalating threat from
H. longicornis [
20]. Ticks are challenging to control due to their rapid rate of reproduction and pesticide tolerance [
21]. Tick vaccines have demonstrated good potential for tick control, considering their safety, the absence of chemical residues, and their sustained effectiveness [
22,
23]. However, it is difficult to dissect these tiny ectoparasites, making it impossible to identify the prospective antigen candidates. Therefore, evaluating already established or freshly characterized proteins as vaccine candidates is a viable strategy.
Enolase is a crucial glycolytic enzyme in the catabolic glycolytic pathway that supplies the energy vital to animals [
24]. In some pathogens, the multifunctional protein functions as a plasminogen receptor to assist their establishment in the hosts [
25]. Previous studies have investigated the role of enolase as a candidate for vaccination against various parasites, including
Ascaris suum [
26] and
Plasmodium falciparum [
27].
In the present study, recombinant enolase protein was produced to examine its impact on ticks. Purified rHlEno may effectively catalyze the conversion of the substrate from 2-PGA to PEP owing to its inherent glycolytic activity. The recombinant enolase protein from E. coli triggered a distinctive, and the effects were observed in mice; the immune response to ticks, particularly the production of protective antibodies, was examined. Statistically significant increases in pre-feeding periods in adult ticks and feeding periods in both larval and adult ticks were observed. Additionally, the attachment rates were significantly reduced in the larval and nymphal ticks. These results suggested that administering the rHlEno protein as a vaccine effectively conferred significant resistance to H. longicornis infestations in mice.
Plasmodium ookinete surface enolase may facilitate the parasite to invade the midguts of mosquitoes [
28]. Moreover, the binding of human plasminogen to enolase from the saliva of
Ornithodoros moubata (Argasid) may play a significant role at the host-tick interface by activating host fibrinolysis and preserving blood fluidity while the ticks are feeding [
29,
30]. Enolase, which is expressed in the salivary gland, is present in the cytoplasm of all eukaryotic cells, where it interacts with the cytoskeleton system to regulate the cell shape and material trafficking; in addition, it plays a role in glycolysis and gluconeogenesis [
31]. Furthermore, enolase helps convert plasminogen to plasmin in the presence of tissue plasminogen activator (tPA) or urokinase plasminogen activator (uPA) [
32,
33].
Plasmodium falciparum enolase antibody was able to block the invasion of merozoite from red blood cells [
33], indicating that enolase may play a major role in the invasion process of host cells.
The expression levels of
H. longicornis enolase were compared across various developmental stages and starvation states. The highest expression was observed in fully engorged adults, and the levels dramatically decreased after 3 or more days of starvation. These findings suggest that enolase may play an important role in plasminogen binding and energy production in tick tissues. Some researchers speculate that enolase may contribute to host-parasite interactions and blood fluidity during feeding [
30], although additional studies are required to support these claims.
Enolase protein implicated in the host protective response to
H. longicornis may be a member of the lyase family. More specifically, it may belong to the hydrolyses, based on the findings of the current study, wherein mice immunized with rHlEno protein demonstrated significant resistance to tick infestation. Based on our hypothesis that the rHlEno protein interacts with tick proteins, we speculated that using specific antibodies to block the rHlEno protein could potentially interfere with the tick invasion process. As a result, ticks feeding on mice with blocked rHlEno would experience enhanced attachment (
Fig. 3) and be able to ingest more blood. This increased blood intake would lead to an increase in the total weight gain and egg-laying capacity compared to those seen in ticks that were fed on animals immunized with the gene-10 fusion protein.
The findings of this study suggested that antibodies targeted to enolase could hinder the transmission of tick-borne infections, resulting in reduced severity of illness in mice compared to those without such antibodies. Additionally, the recombinant protein rHlEno derived from H. longicornis enolase may be a promising candidate for the development of a tick vaccine owing to its vital role in the blood-feeding process. Notably, this is the first study to demonstrate the impact of antibodies raised against a tick antigen on the survival of tick larvae in vaccinated and infected mice.
In this study, recombinant enolase expressed in
E. coli induced a protective immune response against ticks in mice, evidenced by the prolonged feeding time required during the larval and adult stages (
Fig. 2) and the decrease in the engorged rates in larval ticks (
Fig. 6). These findings imply that mice with the rHlEno protein vaccine developed a high level of resistance to
H. longicornis infections. No significant differences in egg weights or molting periods were observed between adult ticks that fed on rHlEno-immunized mice versus those that fed on control mice. However, ticks fed on rHlEno-immunized mice tended to have lower egg weights than those in the control group.
Finally, antibodies against the rHlEno antigen had an overall efficacy of 90% in the immunized mice, thus indicating that the efficacy of this protein as a vaccine is comparable to that of BM86 (79% efficacy) and another acidic peptide derived from the ribosomal protein P0 (96% efficacy) [
34]. The effects of a recombinant vaccine remain specific parameters of ticks.
This study revealed that mice immunized with enolase protein exhibited significant resistance to tick infestation, thus suggesting that the rHlEno protein may play a crucial role in the host protective responses against H. longicornis. Furthermore, rHlEno protein may be involved in the attachment and blood-sucking processes in H. longicornis ticks. Thus, it may be considered as a potential candidate antigen for the developing tick vaccines against H. longicornis infections in mice.
Notes
-
Author contributions
Conceptualization: Haque MS
Investigation: Haque MS
Methodology: Haque MS
Resources: Haque MS, You MJ
Supervision: You MJ
Visualization: Islam MS
Writing – original draft: Haque MS,
Writing – review & editing: Islam MS, You MJ
-
The authors have no conflicts of interest to declare.
Supplementary Information
Acknowledgements
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agriculture, Food and Rural Affairs Convergence Technologies Program for Educating Creative Global Leader, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant number: 320005-4).
Fig. 1Comparison of the pre-feeding periods (days) during the different developmental stages (larva, nymph, and adult) of the H. longicornis ticks in mice immunized with rHlEno and the gene 10 protein (control). A significant difference (***P<0.0001) in pre-feeding periods was observed between the 2 groups. Data is expressed as the mean±SEM. ns, not significant.
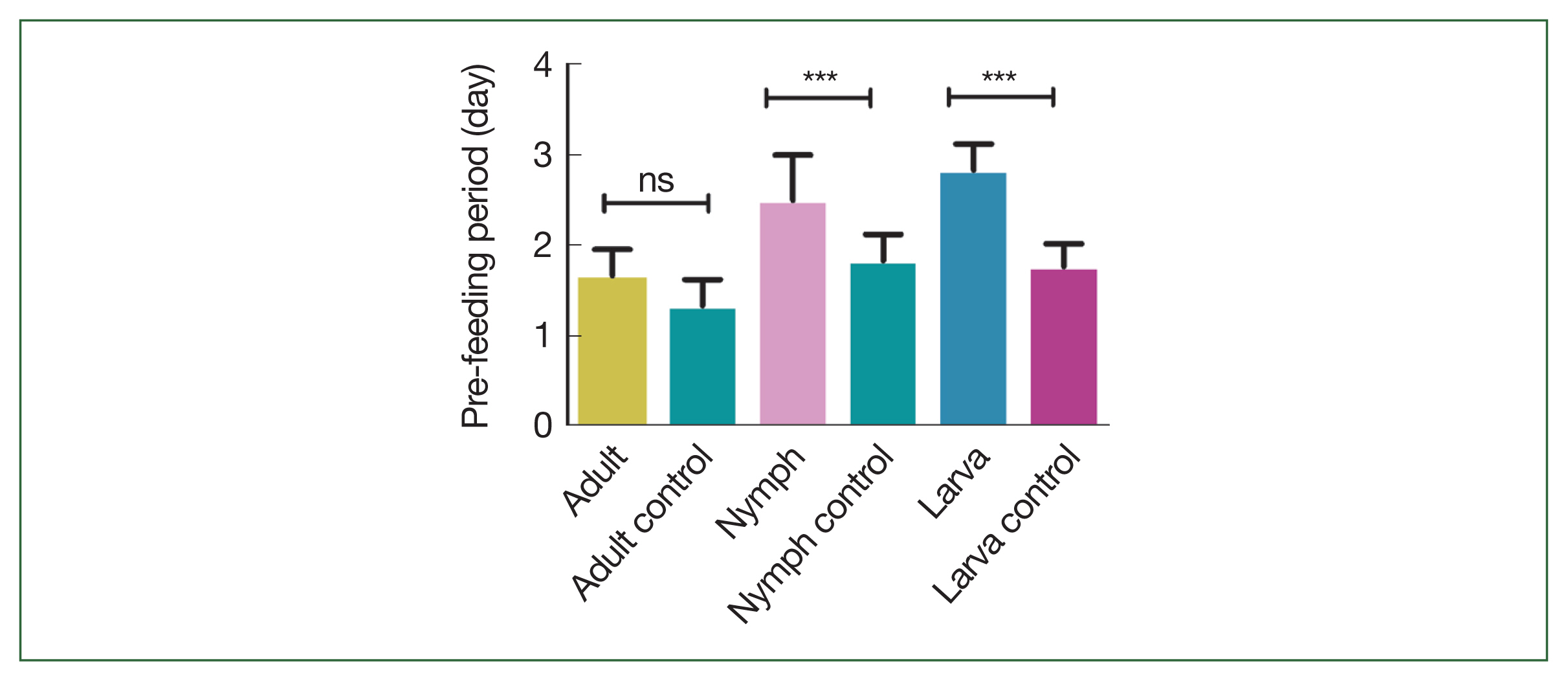
Fig. 2Comparison of the feeding periods during the different developmental stages of the H. longicornis ticks in the 2 group mice immunized with rHlEno and the gene 10 protein (control). Significant differences (*P<0.05) in feeding periods were observed between the 2 groups infested with adult ticks. No significant differences in feeding periods were observed between the 2 groups infested with nymph ticks (ns). Significant differences (*P<0.05) in feeding periods were observed between the 2 groups infested with larval ticks.
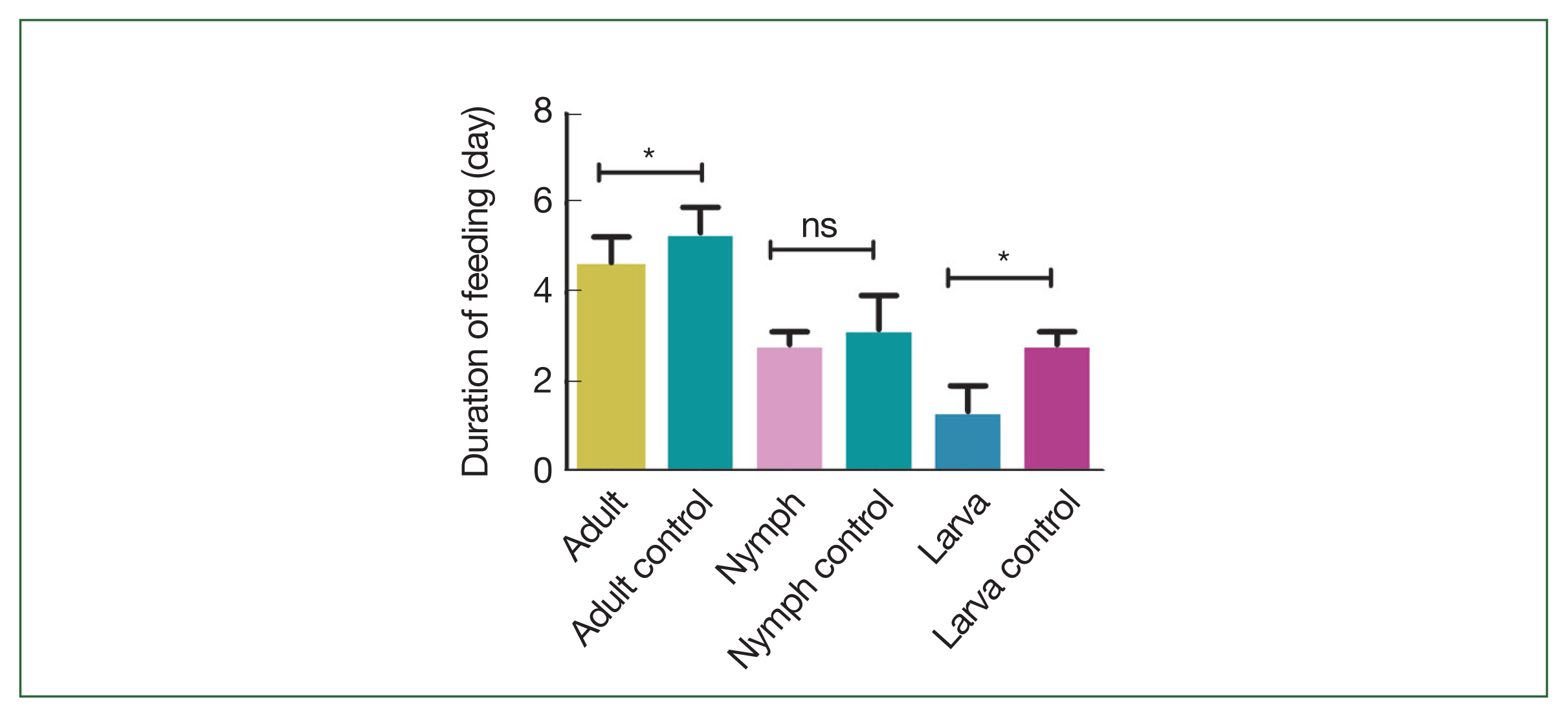
Fig. 3Comparison of attachment rates during the developmental stages of H. longicornis ticks in the rHlEno protein and control groups. Adult ticks attached of these 2 distinct mice groups and compared to control. No significant difference in feeding periods between these groups. Nymph ticks attached of these 2 distinct groups of mice showed no significant difference in feeding periods compared to control groups, but larvae ticks attached to these 2 mice groups showed a highly significant difference (***P<0.001).

Fig. 4Comparison of the death rates (%) after 3 days of infestation with the nymph and larva of H. longicornis ticks in the rHlEno protein and control groups. Significant differences (***P<0.001) in the death rates of the nymphs were observed between the 2 groups. A significant difference (***P<0.001) was also observed during the larval stage.
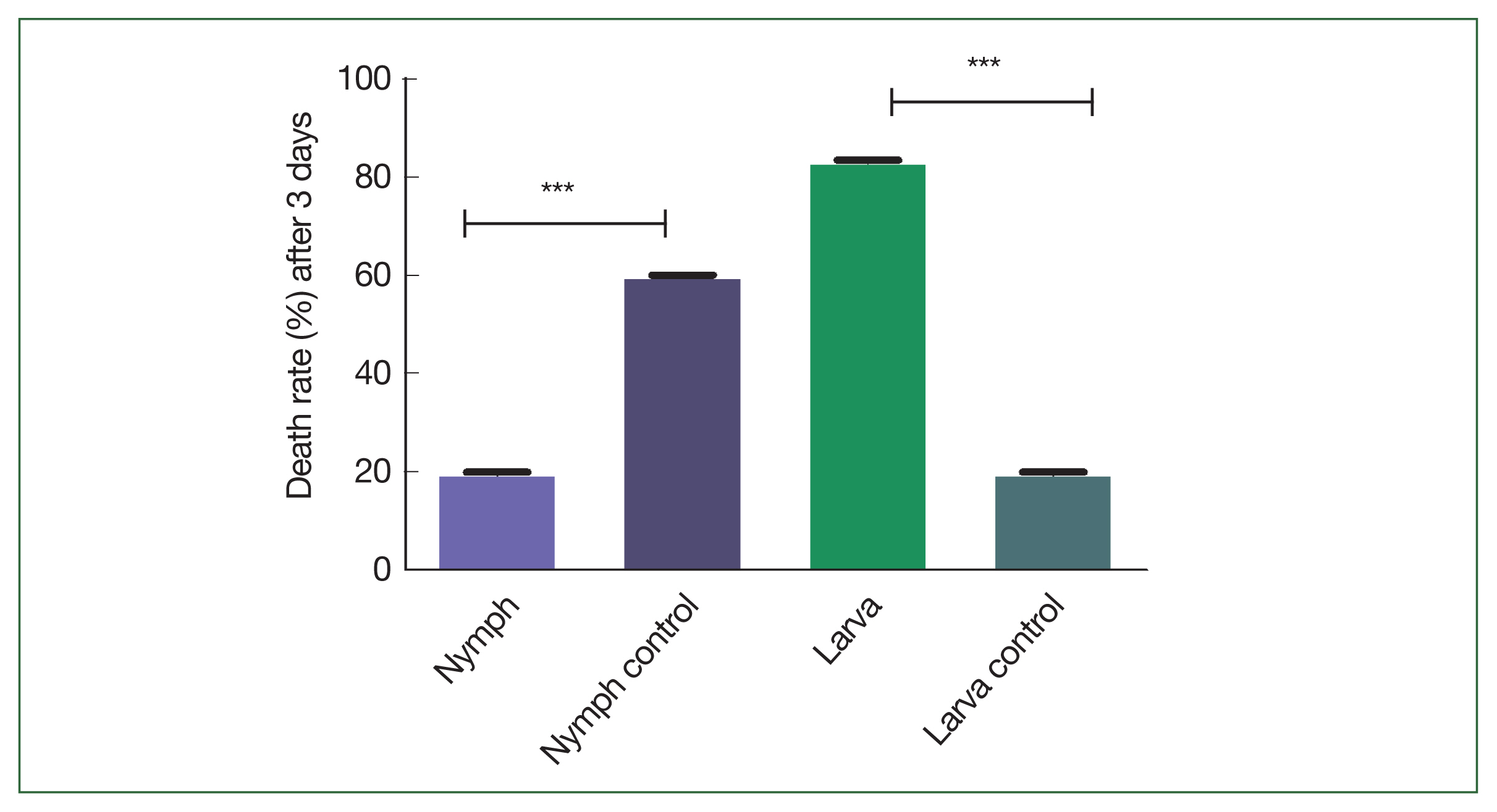
Fig. 5Comparison of the spontaneous dropdown of the adult, nymph, and larva ticks after 3 days of feeding in the immunized mice. Significant differences were observed (***P<0.001).
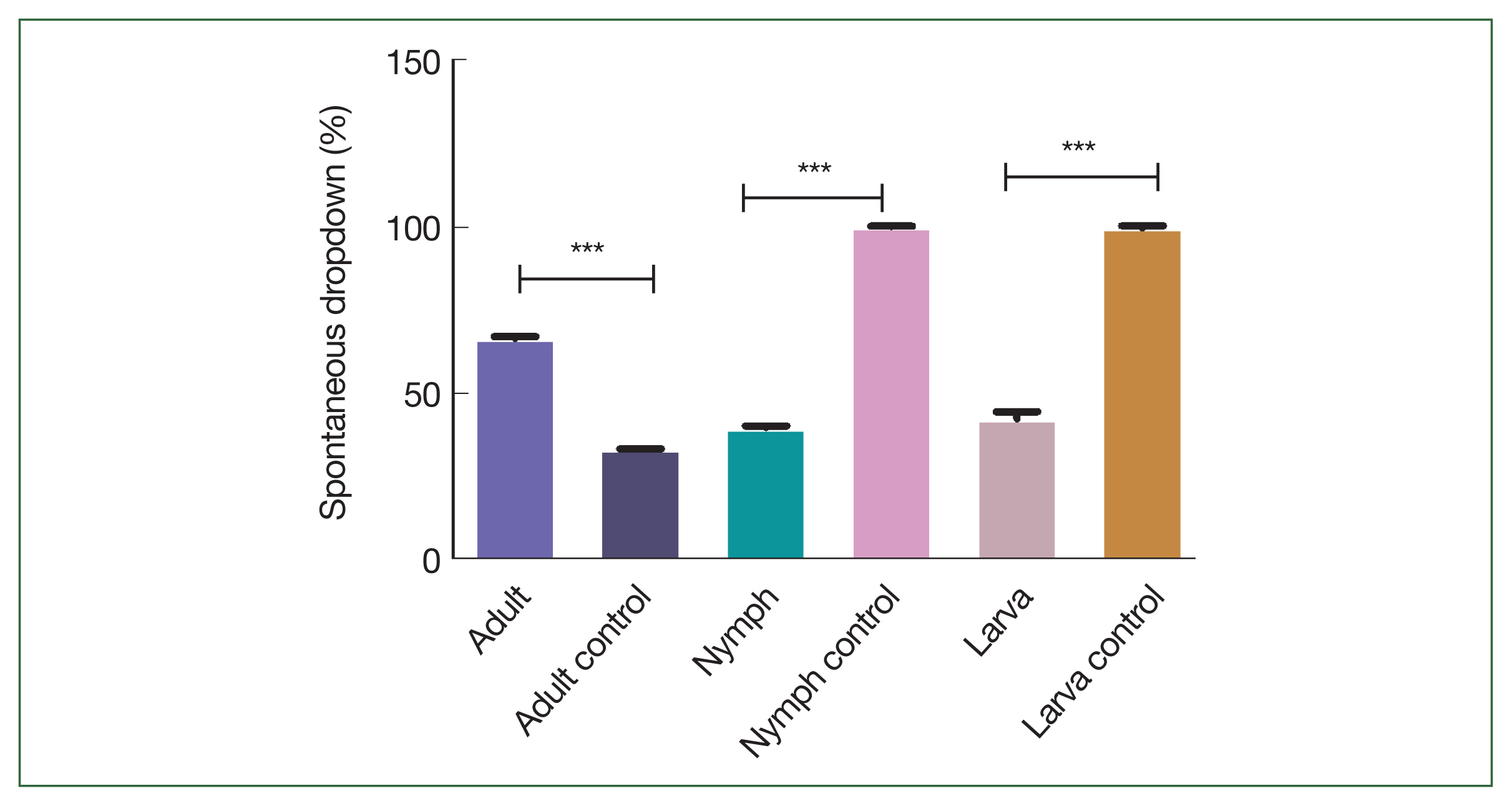
Fig. 6Phenotypic changes during the different developmental stages of H. longicornis ticks immunized with the rHlEno protein and control groups of mice. (A) Adult ticks attached to the rHlEno protein group. (B) Low engorgement of ticks after 6 days of immunization in the rHlEno protein group. (C) Adult ticks attached to the gene-10 protein (control) mice. (D) Engorgement of ticks after 7 days of immunization in the control mice. (E) The nymphs could not attach to the rHlEno protein group on day 3 after immunization. (F) The nymphs in the control group were properly attached on day 3. (G) Larvae could not attach to the rHlEno protein group 3 days after immunization. (H) The larvae in the control group were properly attached on day 3.
References
- 1. Fujisaki K. Development of acquired resistance precipitating antibody in rabbits experimentally infested with females of Haemaphysalis longicornis (Ixodoidea: Ixodidae). Natl Inst Anim Health Q (Tokyo) 1978;18(1):27-38.
- 2. Fujisaki K, Kawazu S, Kamio T. The taxonomy of the bovine Theileria spp. Parasitol Today 1994;10(1):31-33.
https://doi.org/10.1016/0169-4758(94)90355-7
- 3. Wikel SK, Osburn RL. Immune responsiveness of the bovine host to repeated low-level infestations with Dermacentor andersoni
. Ann Trop Med Parasitol 1982;76(4):405-414.
https://doi.org/10.1080/00034983.1982.11687563
- 4. Wikel SK, Whelen AC. Ixodid-host immune interaction. Identification and characterization of relevant antigens and tick-induced host immunosuppression. Vet Parasitol 1986;20(1–3):149-174.
https://doi.org/10.1016/0304-4017(86)90098-1
- 5. Willadsen P. Immunity to ticks. Adv Parasitol 1980;18:293-311.
https://doi.org/10.1016/s0065-308x(08)60402-9
- 6. Nikpay A, Nabian S. Immunization of cattle with tick salivary gland extracts. J Arthropod Borne Dis 2016;10(3):281-290.
- 7. Popara M, Villar M, Mateos-Hernández L, de Mera IG, Marina A, et al. Lesser protein degradation machinery correlates with higher BM86 tick vaccine efficacy in Rhipicephalus annulatus when compared to Rhipicephalus microplus.
. Vaccine 2013;31(42):4728-4735.
https://doi.org/10.1016/j.vaccine.2013.08.031
- 8. de la Fuente J, Rodríguez M, Montero C, Redondo M, García-García JC, et al. Vaccination against ticks (Boophilus spp.): the experience with the Bm86-based vaccine GavacTM
. Genet Anal 1999;15(3–5):143-148.
https://doi.org/10.1016/s1050-3862(99)00018-2
- 9. de la Fuente J, Estrada-Pena A, Venzal JM, Kocan KM, Sonenshine DE. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci 2008;13:6938-6946.
https://doi.org/10.2741/3200
- 10. Kusakisako K, Miyata T, Fujisaki K, Tanaka T. Host immunization with recombinant tick antigen and evaluation of host immune response. Methods Mol Biol 2022;2411:331-341.
https://doi.org/10.1007/978-1-0716-1888-2_19
- 11. Carreón D, de la Lastra JM, Almazán C, Canales M, Ruiz-Fons F, et al. Vaccination with BM86, subolesin and akirin protective antigens for the control of tick infestations in white tailed deer and red deer. Vaccine 2012;30(2):273-279.
https://doi.org/10.1016/j.vaccine.2011.10.099
- 12. Parthasarathi BC, Kumar B, Ghosh S. Current status and future prospects of multi-antigen tick vaccine. J Vector Borne Dis 2021;58(3):183-192.
https://doi.org/10.4103/0972-9062.321739
- 13. Andreotti R. Performance of two Bm86 antigen vaccin formulation against tick using crossbreed bovines in stall test. Rev Bras Parasitol Vet 2006;15(3):97-100.
- 14. Maruyama SR, Garcia GR, Teixeira FR, Brandão LG, Anderson JM, et al. Mining a differential sialotranscriptome of Rhipicephalus microplus guides antigen discovery to formulate a vaccine that reduces tick infestations. Parasit Vectors 2017;10(1):206.
https://doi.org/10.1186/s13071-017-2136-2
- 15. de la Fuente J, Almazán C, Canales M, Pérez de la Lastra JM, Kocan KM, Willadsen P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim Health Res Rev 2007;8(1):23-28.
https://doi.org/10.1017/s1466252307001193
- 16. Riding GA, Jarmey J, McKenna RV, Pearson R, Cobon GS, Willadsen P. A protective “concealed” antigen from Boophilus microplus. Purification, localization, and possible function. J Immunol 1994;153(11):5158-5166.
- 17. Sahibi H, Rhalem A, Barriga OO. Comparative immunizing power of infections, salivary extracts, and intestinal extracts of Hyalomma marginatum marginatum in cattle. Vet Parasitol 1997;68(4):359-366.
https://doi.org/10.1016/s0304-4017(96)01082-5
- 18. Mi R, Yang X, Huang Y, Cheng L, Lu K, et al. Immunolocation and enzyme activity analysis of Cryptosporidium parvum enolase. Parasit Vectors 2017;10(1):273.
https://doi.org/10.1186/s13071-017-2200-y
- 19. You M, Xuan X, Tsuji N, Kamio T, Igarashi I, et al. Molecular characterization of a troponin I-like protein from the hard tick Haemaphysalis longicornis
. Insect Biochem Mol Biol 2001;32(1):67-73.
https://doi.org/10.1016/s0965-1748(01)00081-9
- 20. Tiffin HS, Rajotte EG, Sakamoto JM, Machtinger ET. Tick control in a connected world: challenges, solutions, and public policy from a United States border perspective. Trop Med Infect Dis 2022;7(11):388.
https://doi.org/10.3390/tropicalmed7110388
- 21. Obaid MK, Islam N, Alouffi A, Khan AZ, da Silva Vaz I Jr, et al. Acaricides resistance in ticks: selection, diagnosis, mechanisms, and mitigation. Front Cell Infect Microbiol 2022;12:941831.
https://doi.org/10.3389/fcimb.2022.941831
- 22. Bouazzaoui A, Abdellatif AAH, Al-Allaf FA, Bogari NM, Al-Dehlawi S, et al. Strategies for vaccination: conventional vaccine approaches versus new-generation strategies in combination with adjuvants. Pharmaceutics 2021;13(2):140.
https://doi.org/10.3390/pharmaceutics13020140
- 23. Merino O, Antunes S, Mosqueda J, Moreno-Cid JA, Pérez de la Lastra JM, et al. Vaccination with proteins involved in tick-pathogen interactions reduces vector infestations and pathogen infection. Vaccine 2013;31(49):5889-5896.
https://doi.org/10.1016/j.vaccine.2013.09.037
- 24. Díaz-Ramos A, Roig-Borrellas A, García-Melero A, López-Alemany R. α-Enolase, a multifunctional protein: its role on pathophysiological situations. J Biomed Biotechnol 2012;2012:156795.
https://doi.org/10.1155/2012/156795
- 25. Ayón-Núñez DA, Fragoso G, Bobes RJ, Laclette JP. Plasminogen-binding proteins as an evasion mechanism of the host’s innate immunity in infectious diseases. Biosci Rep 2018;38(5):BSR20180705. https://doi.org/10.1042/bsr20180705.
- 26. Chen N, Yuan ZG, Xu MJ, Zhou DH, Zhang XX, et al.
Ascaris suum enolase is a potential vaccine candidate against ascariasis. Vaccine 2012;30(23):3478-3482.
https://doi.org/10.1016/j.vaccine.2012.02.075
- 27. Dutta S, DasSarma P, DasSarma S, Jarori GK. Immunogenicity and protective potential of a Plasmodium spp. enolase peptide displayed on archaeal gas vesicle nanoparticles. Malar J 2015;14:406.
https://doi.org/10.1186/s12936-015-0914-x
- 28. Ghosh AK, Coppens I, Gårdsvoll H, Ploug M, Jacobs-Lorena M.
Plasmodium ookinetes coopt mammalian plasminogen to invade the mosquito midgut. Proc Natl Acad Sci USA 2011;108(41):17153-17158.
https://doi.org/10.1073/pnas.1103657108
- 29. Díaz-Martín V, Manzano-Román R, Oleaga A, Encinas-Grandes A, Pérez-Sánchez R. Cloning and characterization of a plasminogen-binding enolase from the saliva of the argasid tick Ornithodoros moubata.
. Vet Parasitol 2013;191(3–4):301-314.
https://doi.org/10.1016/j.vetpar.2012.09.019
- 30. Xu XL, Cheng TY, Yang H. Enolase, a plasminogen receptor isolated from salivary gland transcriptome of the ixodid tick Haemaphysalis flava.
. Parasitol Res 2016;115(5):1955-1964.
https://doi.org/10.1007/s00436-016-4938-0
- 31. Keller A, Peltzer J, Carpentier G, Horváth I, Oláh J, et al. Interactions of enolase isoforms with tubulin and microtubules during myogenesis. Biochim Biophys Acta 2007;1770(6):919-926.
https://doi.org/10.1016/j.bbagen.2007.01.015
- 32. Floden AM, Watt JA, Brissette CA.
Borrelia burgdorferi enolase is a surface-exposed plasminogen binding protein. PLoS One 2011;6(11):e27502.
https://doi.org/10.1371/journal.pone.0027502
- 33. Liu X, Zheng C, Gao X, Chen J, Zheng K. Complete molecular and immunoprotective characterization of Babesia microti enolase. Front Microbiol 2017;8:622.
https://doi.org/10.3389/fmicb.2017.00622
- 34. de la Fuente J, Contreras M. Tick vaccines: current status and future directions. Expert Rev Vaccines 2015;14(10):1367-1376.
https://doi.org/10.1586/14760584.2015.1076339