Abstract
Collaborative studies have identified some genetic factors contributing to the development of severe forms of malaria and schistosomiasis. In Thailand, the TNF-α 5'-flanking region shows biallelic polymorphic sites at nucleotides -238, -308, -857, -863, and -1031, and seven alleles have been identified in patients from Myanmar. We found that the TNF promoter (TNFP)-D allele was significantly associated with cerebral malaria in populations from Karen (P < 0.0001, OR = 124.86) and ethnic Burma (P < 0.0001, OR = 34.50). In China, we have identified two major genes related to the severity of liver fibrosis, one an HLA class II gene, and the other the IL-13 gene. The frequency of the HLA-DRB5*0101 allele and that of the IL-13 promoter A/A (IL-13P- A/A) genotype were elevated in fibrotic patients, although the two genes are located on different chromosomes, chromosomes 6p and 5q, respectively. Subjects with both genotypes had odds ratios (OR = 24.5) much higher than the sum of the ratios for each individual genotype (OR = 5.1, 95% Confidence Interval 1.3-24.7 for HLA-DRB5*0101, OR = 3.1 95% CI 1.5 - 6.5 for IL-13P- A/A). That the effects of the two susceptibility markers are synergistic rather than additive, strongly suggests that the pathogenic Th2 response directly influences the prognosis of post-schistosomal liver fibrosis.
-
Key words: Plasmodium falciparum, Schistosoma japonicum, cytokine, HLA, cerebral malaria, schistosomal liver fibrosis
INTRODUCTION
Our recent findings clearly indicate the presence of genetic factors that can predict the prognosis of two major parasitic infectious diseases; falciparum malaria and schistosomiasis japonica (
Hirayama et al., 1999,
Ubalee et al., 2001, Kikuchi et al., In preparation). We are interested in these diseases because they induce serious sequelae, including cerebral malaria and post-schistosomal liver cirrhosis. People in the areas in which these diseases are endemic suffer from both these types of complications. The identification of genes associated with susceptibility or resistance to the severe forms of these diseases will not only be useful for disease prognosis, but also increase our understanding of their pathogenesis, and assist in devising new treatments.
To identify the genes responsible, two different approaches are generally undertaken: targeted gene analysis and genome-wide survey. We adopted the former method, focusing especially on immunity-related genes such as those for HLA, cytokines, and adhesion molecules. HLA genes are highly polymorphic and their alleles are well characterized at the DNA sequence level. For example, the HLA-DRB1 gene has over 100 alleles in the human population. In particular, HLA-DR, -DQ, -DP, -A, -B, and -C are believed to function as immune-response proteins directed against exogenous pathogens. Cytokines are also believed to play important roles in controlling the intensity and duration of the immune response. Recently, single nucleotide polymorphisms (SNPs) have been observed very commonly in the promoter regions of some cytokines, including tumour necrosis factor (TNF), interleukin (IL)-4, IL-13, and interferon-γ. Several studies have demonstrated that these polymorphisms directly affect promoter activity.
We have serious and enthusiastic collaborators in the areas in which these diseases are endemic, and initiated difficult but very interesting studies several years ago. We found surprisingly strong associations between these markers and the diseases.
TNF-α PROMOTER POLYMORPHISM AND CEREBRAL MALARIA
The most severe complication of
Plasmodium falciparum infection is cerebral malaria (CM) (
WHO, 1998). Little is known about the pathogenesis of this condition (
Cook, 1996). Post-mortem examination of the affected brain shows a typical microembolism, commonly called a sequestration, which consists of infected red blood cells in capillaries in the brain mesenchyma (
Aikawa, 1988). Therefore, this sequestration is considered one of the major sequelae causing the typical neurological disturbances that occur in CM. Because the serum levels of TNF-α are elevated in severe malaria (
Grau et al., 1989), excessive production of TNF-α is suspected to produce conditions optimal for this sequestration in capillaries (
Barbara et al., 1996;
Berendt et al., 1989).
We performed a case control study to analyse the polymorphisms of the HLA and TNF-α promoters of CM patients in Myanmar. A total of 245 unrelated malaria patients were randomly selected, consisting of 145 from Karen and 100 from ethnic Burmese groups. The patients were assessed as either being without complications or as having CM at the Mae Sot Malaria Clinic or at the Mae Sot General Hospital using World Health Organization (WHO) criteria (
1990).
A 1,042-bp DNA fragment spanning the 5'-flanking region of the TNF-α gene from positions -66 to -1,107 was amplified by PCR. With hybridization, seven possible combinations of polymorphic sites in the TNF-α promoter (TNFP) were determined in the present study population, giving alleles designated as TNFP-A, -B, -C, -D, -M1, -M4, and -M7. PCR products from each tentative genotype were cloned and sequenced to confirm the alleles. The DNA typing of the HLA-DRB1 gene was performed with the PCR-sequence-specific oligonucleotide probes method used at the XI
th HLA workshop (
Kimura et al., 1992). HLA-B DNA typing was performed as previously described (
Yoshida et al., 1992).
Table 1 shows the seven alleles detected in the present study. The TNFP-A, -B, -C, and -D alleles are identical to those reported in the previous study, which were identified in a Japanese population (
Higuchi et al., 1998;
Skoog et al., 1999). The remaining three alleles, TNFP-M1, -M4, and -M7, are novel alleles that have not been reported elsewhere.
Table 2 shows the allele frequencies in the TNFP region in patients with CM and in those without complications in Karen and ethnic Burmese. The occurrence of the TNFP-D +/+ and +/- genotypes was significantly elevated in patients with CM in both ethnic groups.
This is the first study to report a positive association between the TNFP-D allele and CM. The first report of a relationship between a polymorphism in the TNFP and CM was published by McGuire et al. (
1994), who used the Nco1 restriction enzyme polymorphism (-308A/G) to show that the TNF2 (-308A) allele was associated with CM in Gambian school children. The nucleotide -576 polymorphic site, at which the nuclear factor OCT-1 binds, is associated with CM in the same Gambian population (
Knight et al., 1999). We have also examined this region using specific probes and DNA sequencing, but have found no polymorphisms in Myanmar subjects.
The TNFP-D allele, which we found to be strongly associated with CM in Myanmar, expressed TNF-α more strongly than TNFP-B or -C in studies of peripheral blood mononuclear cells stimulated with concanavalin A in vitro, and in luciferase assays with the Raji cell transfection system (
Higuchi et al., 1998). Skoog et al. (
1999) reported that the NF-κB binding motif is disrupted in the TNFP-B allele by the substitution of -863C with A. This allele is common in Myanmar but is rare in Sweden (
Skoog et al., 1999), and the mutation results in reduced promoter activity. Serum levels of TNF-α were lower in healthy volunteers who were homozygous for this mutant type in Sweden (
Skoog et al., 1999). Although little information is available, when we compared the frequencies of the TNFP alleles in different ethnic groups, we found that frequencies of the -863C or -1031T alleles, both of which are typical of TNFP-D, were much higher in Sweden (0.94 for -863C, 0.80 for -1031T) (
Skoog et al., 1999) and in Japan (0.82 for -863C, 0.84 for -1031T) (
Higuchi et al., 1998) than in Myanmar patients (0.08 for -863C, 0.15 for -1031T). It would be interesting to examine the frequencies of these alleles in African populations. It is possible for natural selection to generate such differences by natural selection.
POLYMORPHISMS IN HLA CLASS II GENES AND THE IL-13 PROMOTER AND SCHISTOSOMAL LIVER FIBROSIS
Schistosomiasis japonica is a chronic helminthic infectious disease that affected at least 860,000 individuals in China in 1995. Morbidity and mortality are dependent on its chronic sequelae, post-schistosomal hepatosplenic disease, which is characterized by liver fibrosis, portal hypertension, ascites accumulation, oesophageal varices, and eventual death. The liver fibrosis seen in these patients is induced by a granulomatous immune response against the eggs that are deposited in the periportal area (
Cheever et al., 1993). Schistosomal egg antigen-specific CD4+ T cells play a major role in the formation of granuloma through Th2-type cytokine production in experimental schistosomiasis mansoni (
Wynn et al., 1995;
Kaplan et al., 1998). However, in humans, little is known about the immunological response during the chronic phase of hepatosplenic disease (
Wiest et al., 1992). Because only 5-10 per cent of patients with chronic schistosomiasis japonica develop hepatosplenic disease, and because the granulomatous response is initiated by CD4+ T cells reactive to schistosomal antigen, polymorphisms of the HLA class II antigens, which control the reactivity of the CD4+ T cells, may be associated with a susceptibility to hepatosplenic disease. Indeed, associations between schistosomal hepatosplenic disease and HLA alleles have been reported for schistosomiasis mansoni (
Salam et al., 1979) and for schistosomiasis japonica (
Ohta et al., 1982;
Ohta et al., 1989). Recently, more objective diagnostic methods using ultrasonography have become popular and have been standardized to measure changes in liver morphology (
The Cairo Working Group, 1992). Therefore, we used this method to categorize the patients into a "fibrotic" group and a "non-fibrotic" group, and examined their genetic characteristics by analysing the polymorphisms of candidate genes encoding HLA class II and class I antigens, TNF-α and cytokines.
A total of 230 current or former patients with chronic schistosomiasis japonica were examined for liver changes. All patients were from the agricultural village of Beishan, in Yushan county, China, and had their first episode of infection and treatment at least 10 years before this study began. The mean age of the subjects was 52.6 ± 10.5 years and the mean time since their initial treatment was 27.4 ± 8.8 years. Ultrasonographic diagnosis was carried out according to the WHO standard for the diagnosis of liver fibrosis due to schistosomiasis japonica (
The Cairo Working Group, 1992;
Hatz et al., 1992;
Hirayama et al., 1998) (
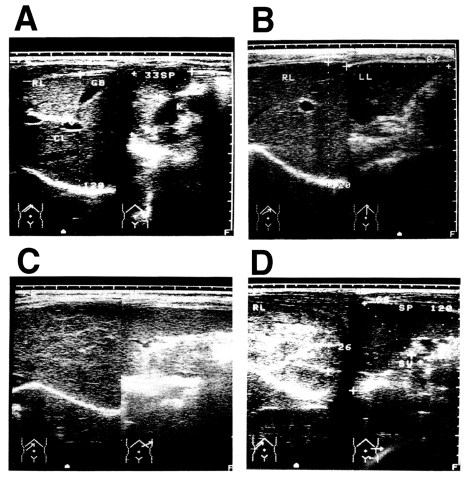
Fig. 1). Ultrasonographic diagnosis determined that there were 44 persons with grade 0 fibrosis, 81 with grade I fibrosis, 99 with grade II fibrosis, and six with grade III fibrosis. The presence of hepatitis B virus (HBV) was not assessed in these patients, but the prevalence of HBV is about 15% in Jiangxi Province (
Li et al., 1997). Most of the men in the village smoke tobacco and drink alcoholic beverages, but the women generally do not. The patients had all been treated with praziquantel after each positive faecal examination throughout their lives, but it was not possible to estimate the precise total worm burden of each patient during the clinical course of the disease. Therefore, we tentatively defined an appropriately exposed person as someone with a record of repeated treatments for schistosomiasis japonica over a 10-year period (
Hirayama et al., 1998).
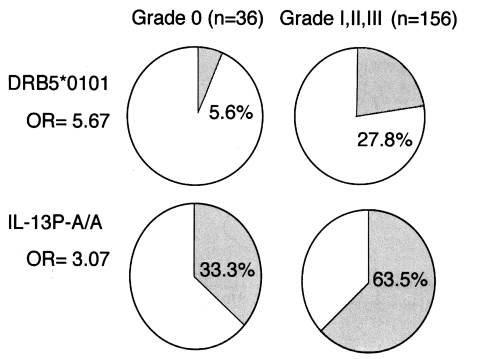
In total, 29 alleles for HLA-DRB1, three for HLA-DRB3, two for HLA-DRB5, 13 for HLA-DQA1, 11 for HLA-DQB1, four for HLA-DPA1, 18 for HLA-DPB1, and 24 for HLA-B were detected in this population. No significant difference in the frequency of the HLA-B types was observed in the two groups defined by the severity of their fibrosis. On the contrary, the frequencies of several HLA class II alleles were significantly increased or decreased in the fibrotic groups. When we compared the frequencies of alleles between grade 0 and grades I, II, and III, we found that HLA-DRB1*1101 (P < 0.001), DQA1*0501 (P < 0.02), and DQB1*0301 (P < 0.03), which are closely linked, were significantly elevated in the grade 0 group, and that HLA-DRB5*0101 was significantly elevated in grade I, II, and III fibrotic patients (P < 0.03) (
Fig. 2). This suggests that the HLA-DRB1*1101-DQB1*0301-DQA1*0501 haplotype (P < 0.02) decreases susceptibility to grades I, II, and III fibrosis, whereas the HLA-DRB1*1501-DRB5*0101 haplotype (P < 0.02) increases this susceptibility. If we assume that these genetic associations arise from the functions of the HLA molecules themselves, then the critical question is: How do these molecules present antigens to CD4+ T cells to initiate the immunological processes leading to fibrosis? We have not yet identified the antigen(s) responsible for the stimulation of pathogenic or protective T cells via such HLA molecules.
We analysed the polymorphisms of the Th2 cytokine genes in the same subjects. In these subjects, there was a significant association between homozygosity for the IL-13P polymorphism and the liver fibrosis group, as shown in
Fig. 3. Because the IL-13 gene is localized on the long arm of chromosome 5, the IL-13P allele must be inherited independently of the HLA class II allele that occurs on the short arm of chromosome 6. Therefore, the next question is whether there is any interaction between these two genetic markers, HLA and IL-13. As shown in
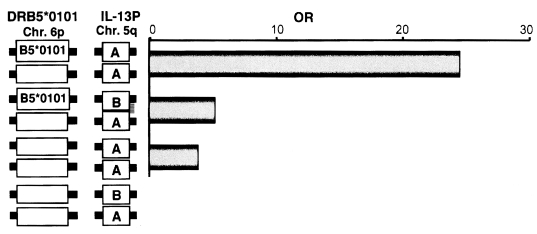
Fig. 3, both HLA-DRB5*0101- and IL-13P- A/A-positive subjects had much higher odds ratios (OD = 24.5) than subjects positive for only one of these polymorphisms (OD = 5.1 for HLA, OD = 3.7 for IL-13P- A/A), indicating these two genetic markers synergistically enhance the development of fibrosis after infection. This synergy is explained by the cartoon in
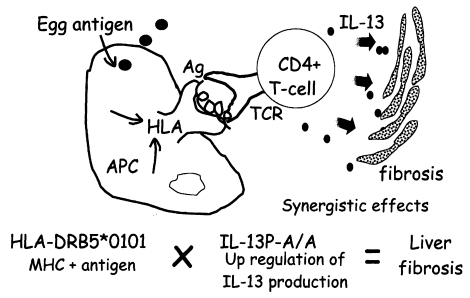
Fig. 4, showing that the pathogenic IL-13-producing CD4+ T cells are preferentially stimulated by the antigen-presenting cells expressing HLA-DRB5*0101. Further study is required to show that this cartoon accurately depicts the phenomenon.
CONCLUSION
A specific polymorphism of the TNF-α promoter profoundly influenced the prognosis of cerebral malaria in adult patients in Myanmar. The synergistic effect of two susceptibility markers, HLA-DR and IL-13P alleles, was clearly demonstrated in the development of liver fibrosis in patients with chronic Schistosoma japonicum infection in China.
Notes
-
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Sports, Science and Culture, Japan, (08281104, 08044316, 09470071, 10044317) and by the Maruki Memorial Research Fund (A) of 1998 of Saitama Medical School.
ACKNOWLEDGEMENTS
Ratawan Ubalee (Nagasaki University), Fumi Suzuki (Saitama Medical School), Mihoko Kikuchi (Nagasaki University), Oumaporn Tasanor (Mahidol University), Yupaporn Wattanagoon (Mahidol University), Ronnatrai Ruangweerayut (Mae Sot General Hospital), Kesara Na-Bangchang (Thammasat University), Juntra Karbwang (WHO), Akinori Kimura (Tokyo Medical & Dental University), Kyogo Itoh (Kurume University), and Tozo Kanda (St. Marianna Medical College), contributed to the malaria study. Chen Honggen, Yin Dong, Gu Xiaonan, and Zhang Shaoji, (Jiangxi Province Institute of Parasitic Diseases), Mihoko Kikuchi (Nagasaki University), and Jianxiang Liu and Hong-Chang Yuan (Shanghai Medical University) contributed to the schistosomiasis study.
References
- 1. Aikawa M. Human cerebral malaria. Am J Trop Med Hyg 1988;39:3-10.
- 2. Barbara JA, Vn Otand X, Lopez A. Tumor necrosis factor-α (TNF-α): The good, the bad, and the potentially very effective. Immunol Cell Biol 1996;74:434-443.
- 3. Berendt AR, Simmons DL, Tansey J, Newbold CI, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature 1989;341:57-59.
- 4. Cheever AW. Schistosomiasis. Infection versus disease and hypersensitivity versus immunity. Am J Pathol 1993;142:699-702.
- 5. Cook GC. Mansons tropical diseases. 1996, Philadelphia, USA. Philadelphia Saunders; pp 1107-1116.
- 6. Grau GE, Taylor TE, Molyneux ME, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med 1989;320:1586-1591.
- 7. Hatz C, Murakami H, Jenkins JM. A review of the literature on the use of ultrasonography in schistosomiasis with special reference to its use in field studies. 3. Schistosoma japonicum. Acta Tropica 1992;51:29-36.
- 8. Higuchi T, Seki N, Kamizono S, et al. Polymorphism of the 5'-flanking region of the human tumor necrosis factor (TNF)-α gene in Japanese. Tissue Antigens 1998;51:605-612.
- 9. Hirayama K, Chen H, Kikuchi M, et al. Glycine-valine dimorphism at the 86th amino acid of HLA-DRB1 influences the prognosis of post-schistosomal hepatic fibrosis. J Infect Dis 1998;177:1682-1686.
- 10. Hirayama K, Chen H, Kikuchi M, et al. HLA-DR-DQ alleles and HLA-DP alleles are independently associated with susceptibility to different stages of post-schistosomal hepatic fibrosis in the Chinese population. Tissue antigens 1999;53:269-274.
- 11. Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol 1998;160:1850-1856.
- 12. Kimura A, Sasazuki T. In Tsuji K, Aizawa M, Sasazuki T eds, Eleventh International Histocompatibility Workshop reference protocol for the HLA DNA-typing technique. HLA 1991 Volume 1. Proceedings of the Eleventh International Histocompatibility Workshop and Conference. 1992, New York, USA. Oxford University Press; pp 397-419.
- 13. Knight JC, Udalova I, Hill AV, et al. A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria. Nat Genet 1999;22:145-150.
- 14. Li Y, Yu DB, Li YS, Ross AG, McManus DP. Infections with hepatitis B virus in three villages endemic for schistosomiasis japonica in the Dongting Lake region of China. Ann Trop Med Parasitol 1997;91:323-327.
- 15. McGuire W, Hill AV, Allsopp CE, Greenwood BM, Kwiatkowski D. Variation in the TNF-α promoter region associated with susceptibility to cerebral malaria. Nature 1994;371:508-511.
- 16. Ohta N, Hayashi M, Tormis LC, Blas BL, Nosenas JS, Sasazuki . T. Immunogenetic factors involved in the pathogenesis of distinct clinical manifestations of schistosomiasis japonica in the Philippine population. Trans R Soc Trop Med Hyg 1987;81:292-296.
- 17. Ohta N, Nishimura YK, Iuchi M, Sasazuki . T. Immunogenetic analysis of patients with post-schistosomal liver cirrhosis in man. Clin Exp Immunol 1982;49:493-499.
- 18. Salam EA, Ishaac S, Mahmoud AA. Histocompatibility-linked susceptibility for hepatosplenomegaly in human schistosomiasis mansoni. J Immunol 1979;123:1829-1831.
- 19. Skoog T, van't Hooft FM, Kallin B, et al. A common functional polymorphism (C→A) substitution at position -863) in the promoter region of the tumor necrosis factor-α (TNF-α) gene associated with reduced circulating levels of TNF-α. Hum Mol Genet 1999;8:1443-1449.
- 20. The Cairo Working Group. The use of diagnostic ultrasound in schistosomiasis: attempts at standardization of methodology. Acta Tropica 1992;51:45-63.
- 21. Ubalee R, Suzuki F, Kikuchi M, et al. Strong association of a tumor necrosis factor-α promoter allele with cerebral malaria in Myanmar. Tissue Antigens 2001;58:407-410.
- 22. Wiest PM, Wu G, Zhang S, et al. Schistosomiasis japonica on Jishan Island, Jiangxi Province, People's Republic of China: persistence of hepatic fibrosis after reduction of the prevalence of infection with age. Trans R Soc Trop Med Hyg 1993;87:290-294.
- 23. World Health Organization. Severe and complicated malaria. Trans R Soc Trop Med Hyg 1990;84(Supplement 2):1-65.
- 24. Wynn TA, Cheever AW, Jankovic D, et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature 1995;376:594-596.
- 25. Yoshida M, Kimura A, Numano F, Sasazuki T. Polymerase-chain-reaction-based analysis of polymorphism in the HLA-B gene. Hum Immunol 1992;34:257-266.
Fig. 1Diagnosis of post-schistosomal liver fibrosis and cirrhosis by ultrasonography. A: Grade 0. B: Grade I. C: Grade II. D: Grade III, showing the fish-scale pattern.
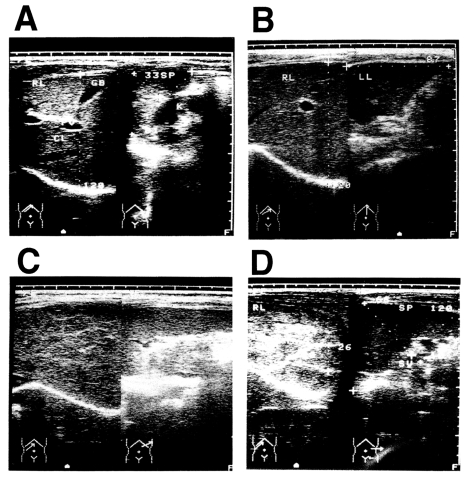
Fig. 2Associations of HLA-DRB5*0101 and IL-13P-A/A with the fibrotic group of patients with chronic schistosomiasis.
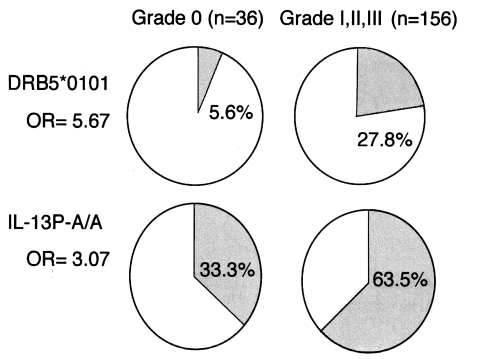
Fig. 3Synergistic effect of the two susceptibility markers, HLA-DRB5*0101 and IL-13P-A/A. OR and 95% CI were calculated relative to individuals negative for both DRB5*0101 and IL-13P-A/A.
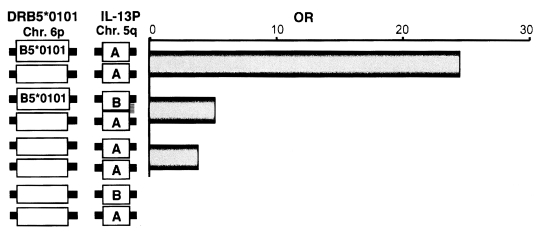
Fig. 4The synergistic effect of two susceptibility markers suggests the presence of pathogenic CD4+ T cells in the liver.
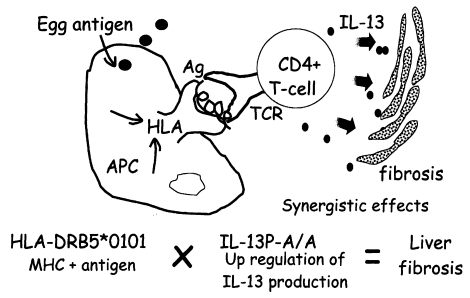
Table 1.TNF-α 5′-flanking region polymorphisms detected in Myanmar patients with malaria
Table 1.
|
Polymorphic sites in the promoter region of the TNF-α gene
|
|
-238 |
-308 |
-857 |
-863 |
-1031 |
|
TNFP-A |
G |
G |
C |
C |
T |
|
TNFP-B |
G |
G |
C |
A |
C |
|
TNFP-C |
A |
G |
C |
C |
C |
|
TNFP-D |
G |
G |
T |
C |
T |
Table 2.Genotype frequencies of the 5′-flanking region of the TNF-α gene in CM patients and patients without complications in Myanmar
Table 2.
|
|
Burmese
|
Karen
|
|
|
No complications
|
CM
|
No complications
|
CM
|
|
Genotype |
|
n = 94 (%) |
n = 21 (%) |
n = 106 (%) |
n = 22 (%) |
|
TNFP-D |
-/- |
92 (97.9) |
12 (57.1) |
106 (100) |
14 (63.6) |
|
+/+ and +/- |
2 (2.1) |
9 (42.9)a)
|
0 (0.0) |
8 (36.4)b)
|