Abstract
The cyclophilins (Cyps) are family members of proteins that exhibit peptidyl-prolyl cis-trans isomerase (PPIase, EC 5.2.1.8) activity and bind the immunosuppressive agent cyclosprin A (CsA) in varying degrees. During the process of random sequencing of a cDNA library made from Giardia intestinalis WB strain, the cyclophilin gene (gicyp1) was isolated. An open reading frame of gicyp1 gene was 576 nucleotides, which corresponded to a translation product of 176 amino acids (Gicyp1). The identity with other Cyps was about 58-71%. The 13 residues that constituted the CsA binding site of human cyclophilin were also detected in the amino acid sequence of Gicyp1, including tryptophan residue essential for the drug binding. The single copy of the gicyp1 gene was detected in the G. intestinalis chromosome by southern hybridization analysis. Recombinant Gicyp1 protein clearly accelerated the rate of cis → trans isomerization of the peptide substrate and the catalysis was completely inhibited by the addition of 0.5 µM CsA.
-
Key words: Giardia intestinalis, cyclophilins, cyclosporin, peptidylprolyl isomerase
INTRODUCTION
Giardia is a diplomonad protozoan parasite that causes intestinal infection in mammals, birds, reptiles, and amphibians. It is an important cause of waterborne and restaurant associated outbreaks of diarrhea, travelers' diarrhea and diarrhea in child care facilities (
Adam, 2001). Because of absence of mitochondria and peroxisome organells present even in other protist and 16S ribosomal RNA sequence to indicate their ancient lineage (
Sogin et al., 1993),
Giardia is thought to be an important organism in evolutionary biology.
Cyclophilin (Cyp) proteins were first characterized in human and bovine spleen, based on their high-affinity binding to the immunosuppressive drug cyclosporin A (CsA) (
Handschumacher et al., 1984). CsA was also shown to possess unexpected antiparasitic activities against schistosomes, plasmodia, cestodes, and nematodes (Chappell and Cha, 1988). Cyp proteins were found to have peptidylproryl
cis-trans isomerase (PPIase) activity, thus catalysing the
cis-trans isomerization of proline imidic bonds in peptides (
Fischer et al., 1989).
Up to now, several cDNAs encoding Cyp proteins have been described. Cyp A, the homolog of the bovine protein, is cytosolic (
Haendler et al., 1987), and Cyp B is found either in secretions or in the endoplasmic reticulum, possibly associated with calciosomes, after cleavage of signal peptide (
Spik et al., 1991). Whereas Cyp C (less abundant form) is localized in the endoplasmic reticulum (
Schneider et al., 1994), and Cyp D is probably mitochondrial (
Bergsma, 1991) and Cyp-40 is found in association with the molecular chaperone Hsp90-steroid receptor complex (
Duina et al., 1998).
The sequence conservation, wide tissue distribution, and organelle targeting of the cyclophilin variants suggest that they possess important functions in many cell compartments. This view is supported by the discovery of cyclophilin homologs in prokariotic cells (
Liu & Walsh, 1990) and various plants (
Gasser et al., 1990).
The diverse effects of CsA reported in several parasitic infections have evoked an interest for Cyps from parasitic species. The CsA exerts an antimalarial activity in vivo and in vitro, and the inhibitory effect on the progress of lesions by
Leishimania species is due to suppression of host T-lymphocyte activities (
Solbach et al., 1986). McCabe et al. (1986) also reported that CsA has antiprotozoal and immunomodulatory activities in
Toxoplasma infection. Recently, a Cyp protein of
Entamoeba histolytica was reported, and it was demonstrated that the trophozoites were susceptible to the treatment with CsA.
Although there are numerous studies on Cyp and CsA action on parasites, no information is available as for G. intestinalis. In the present study, we describe isolation, expression and characterization of a gene coding for Giardia Cyp and CsA action on Giardia trophozoites.
MATERIALS AND METHODS
Giardia culture
Giardia intestinalis WB strain (ATCC 30957) was obtained from American Type Culture Collection and maintained in Keister's modified TYI-S-33 medium, supplemented with 10% fetal bovine serum at 37℃.
Identification and characterization of cDNA clone, gicyp1
In the process of EST's (express sequence tags) construction from a λZAP II cDNA library which was made from trophozoites of G. intestinalis, the cyclophilin gene was serendipitously isolated. The cloned insert, gicyp1, contained 536 bp and was found to be a partial clone. Sequence analysis and homology comparisons were performed using the program of the Genbank blast-search.
5'RACE (rapid amplification of cDNA end) of gicyp1
To obtain a full-length coding sequence of gicyp1, 5'RACE PCR was carried out by using Marathon™ cDNA Amplification Kit (Clontech, Palo Alto, CA USA). PCR product was cloned into PGM-T vector (Promega, Madison, WI, USA) and sequenced using T7 sequencing kit (Amersham Biosciences, Piscataway, NJ, USA).
Hybridizations
The DNA probe was prepared from gicyp1 cDNA clone. For Southern and northern blot analyses, cDNA probe with specific activity of 2-5×108 cpm/µg was made by Random labeling kit (Roche Molecular Biochemicals, Mannheim, Germany) with 32P (3000 Ci/mmol). Aliquots (10 µg/lane) of genomic DNA from G. intestinalis were digested with Pst I, Eco RI and Hind III (Roche). Northern blot analysis was carried out using 10 µg of total RNA prepared from trophozoite. Nucleic acids were separated by electrophoresis and transferred to nitro-cellulose membrane (BioRad, Hercules, USA). The pre-hybridized membranes were incubated for 1hr at 68℃ with 2×106 cpm of nick-translated probes in Express hybridization solution (Clontech).
Construction of Gicyp1 expression vectors in Escherichia coli
Recombinant cyclophilin was expressed in
E. coli using pGEX4T-1 (
Smith and Johnson 1988). A
gicyp1 insert was obtained by complete digestion of
gicyp1 clone with
Eco RI/
Xho I (Posco, Pohang, Korea). This fragment was ligated into pGEX 4T-1 (Amersham Biosciences) that was digested with
Eco RI/
Xho I, and transformed into
E. coli XL1-Blue. By Wisard mini prep (Promega), plasmid DNA was extracted from the ampicillin - containing overnight culture. The plasmid was digested with
Eco RI/
Xho I to release a cDNA insert (original insert size, 0.5 kbp) and retransformed into
E. coli BL21. The overnight culture (200 ml) diluted 1:10 in fresh Luria Bertani media supplemented with ampicillin (50 µg/ml) was grown for 1 hr at 37℃ before the addition of 0.3 mM IPTG. Following 3 hr of growth, cells were pelleted, resuspended in lysis buffer (containing 20 mM Tris pH 7.4, 500 mM NaCl, 10% glycerol, 1% NP-40), and sonicated four times at 15-s intervals. The supernatant was filtered with a 0.45-µm filter and passed over a glutathione-4B Sepharose column (Amersham Biosciences). The column was washed with phosphate-buffered saline (PBS) and eluted with 5 mM reduced-glutathione (Sigma, St. Louis. MI, USA) in 50 mM Tris-HCl (pH 8.0). After the removal of glutathione by filtration, the product was cleaved with human thrombin (Sigma) (25℃, 12 h) as described by Smith and Johnson (
1988). The fusion protein, Gicyp1, was removed by passage over a glutathione-Sepharose column and the recombinant protein was analyzed by SDS-polyacrylamide gel electrophoresis.
PPIase activity of Gicyp1 was assayed in a Shimadzu UV-1201 spectrophotometer (Shimadzu, Japan), essentially as described (
Fischer et al., 1989), with slight modification to measures the
cis to
trans isomerization of alanine proline peptide bond in the synthetic peptide
N-succinyl-Ala-Ala-Pro-Phe-
p-nitroanilide (Sigma). Test peptide (2.1 mM)
N-succinyl-Ala-Ala-Pro-Phe-
p-nitroanilide was dissolved in methanol (Standaert et al., 1990), and α-chymotrypsin (2 mg/ml in 100 mM Tris-HCl, pH 8.0) (Sigma) was added following preincubation of PPIase with the test peptide at 0℃. The reaction mixture (1 ml) containing the test peptide (63 µM), chymotrypsin (0.06 mg/ml), and varying amounts of Gicyp1 was placed into a spectrophotometer cell at 12℃. The first reading was taken at 5 sec to account for the dead time in the mixing of the sample, and readings were taken at 10-sec intervals. All reactions were performed in triplicate. For inhibition assay of CsA on PPIase activity of recombinant Gicyp1 protein, 0.5 µM CsA was added to the above PPIase activity test.
RESULTS
Cloning of G. intestinalis cyclophilin gene
The partial cDNA clone Gi054 encoding the
G. intestinalis cyclophilin-like protein lacked a putative initiation codon ATG triplet. The 3' end of this clone had TAA stop codon and a putative polyadenylation signal AGTAAA (
Fig. 1). To obtain the complete 5' end of this gene, 5' RACE PCR amplification was performed on mRNA prepared from trophozoites using reverse primers constructed around the 5' region of the
gicyp1 sequence. Sequencing of an amplification product revealed a 174 bp extension at the 5' end of this clone (
Fig. 2). An ORF (open reading frame) of
gicyp1 gene was 576 nucleotides, which corresponds to a translated product of 176 amino acids. The upstream sequence was A + T rich as has been reported for other giardial genes (
Minotto et al., 1999). The sequence TATAA, a putative TFIID/TBP binding element, was located ≈ 60 bp upstream from the start codon (
Fig. 1). The sequence CAAAT box occurred two times within 90 bp upstream from the start codon (
Fig. 1) that has been reported in the upstream sequences in other giardial genes (
Minotto et al., 1999).
The FASTA homology comparison program of the BLAST search revealed a high degree of homology between
gicyp1 and cyclophilins of many other species. The identity with other Cyps was 58-71%. Gicyp1 shared 71% and 64% amino acid identity with
E. histolytica cyclophilin (AF017993) and human cyclophilin (X52851), respectively. The predicted amino acid sequence has a calculated molecular mass of 19,005 Da. One motif YkgSxFHRXIpkFMiQGG (amino acid 59-76) corresponded to the Cyclophilin-type peptidyl-prolyl cis-trans isomerase signature in the Prosite library of sequences. The 13 residues that constitute the cyclosporin (CsA) binding site of human cyclophilin were also detected in the amino acid sequence of Gicyp1, including tryptophan residue essential for the drug binding (
Liu et al., 1991) (
Fig. 2).
Genomic DNA of
G. intestinalis WB strain was digested with three restriction enzymes, and the digests were separated on 1% agarose gel and transferred to nitrocellulose membrane. The filter was probed with the radioactively labeled partial
gicyp1 cDNA. As shown in
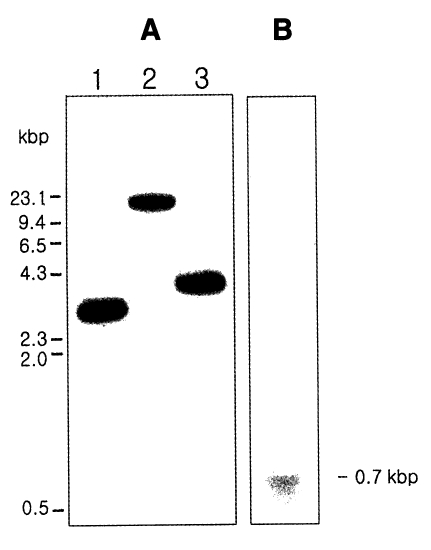
Fig. 3A, the result of the hybridization showed the presence of one large DNA band between 4 and 9 kb.
Total RNA was isolated from
G. intestinalis trophozoites and analyzed by Northern blotting with the radiolabeled 500-bp cDNA insert as a probe (
Fig. 3B). The positive signal of approximately 700 bp consisted of a cDNA coding sequence of 576 bp, 5' untranslated region, 3' noncoding region, and a poly A tract.
Gicyp1 clone was digested with
Eco RI/
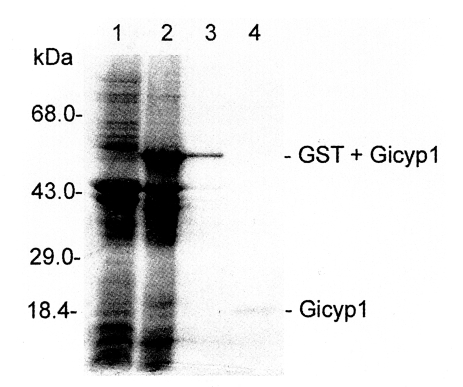
Xho I, and the insert was cloned into the prokaryotic expression vector pGEX4T-1 (pGX-Gicyp1). As revealed by 12% SDS-PAGE (
Fig. 4), the recombinant protein was expressed as a fusion protein of
G. intestinalis Gicyp1 and glutathione-S-transferase with an expected size of 57 kDa. GST fusion recombinant protein was cleaved with thrombin, and the resulting protein was purified on a glutathione-sepharose column and found to be Mr = 20 kDa size (
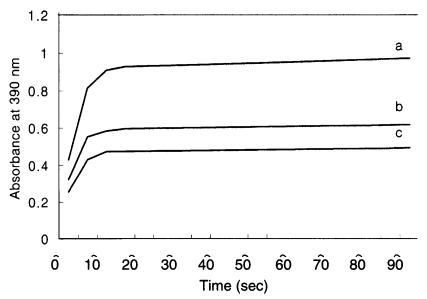
Fig. 4). Purified recombinant Gicyp1 was assayed for PPIase activity. The isomerization of the Ala-Pro bond coupled with chymotryptic cleavage of the trans peptide was measured by the increase of absorbance at 390 nm. Recombinant Gicyp1 protein clearly accelerated the rate of
cis →
trans isomerization of the peptide substrate relative to the control and the catalysis was completely inhibited by the addition of 0.5 µM CsA (
Fig. 5).
DISCUSSION
Cyclophilin coding gene
gicyp1 was identified in
G. intestinalis for the first time. The incomplete cDNA clone Gi054 lacked 5' untranslated regions and start codon but had complete 3' region including the stop codon and polyadenylation signal. Through 5' RACE PCR, complete sequences of 5' region were obtained. Yee et al. (
2000) showed that the GDH promoter with AT-rich region at the initiation site as well as a CAAAT region 34 bp upstream was important. The results of 5' RACE PCR revealed that the
gicyp1 gene also had this promoter region (
Fig. 1). A motif resembling the putative AGTRAAY polyadenylation signal, often found in giardial genes (
Minotto et al., 1999), was found 6 bp downstream from stop codon and 6 bp upstream from poly A sequences. These results are in agreement with the typically short 5' and 3' untranslated regions reported for other
Giardia genes (
Adam, 2001). The full-length coding sequence of Gicyp1 revealed 71% amino acid identity with previously reported
Entamoeba cyclophilin EhCyp (
Fig. 2). This extent of identity with EhCyp was higher than those of other parasites and some mammal Cyp sequences, the result being in agreement with the evolutionary lineage of
Giardia and
Entamoeba, which lack mitochondria. The Gicyp1 recombinant protein has deduced molecular weight of 19 kDa, which is within the range of other Cyps. The Cyp signature motif and residues involved in CsA binding were detected in Gicyp1 putative amino acid sequence. Also, the functional properties of the purified recombinant Gicyp1 demonstrated that it possessed PPIase activity and this activity was inhibited in vitro by CsA. The PPIase activity could be related with FK506 binding protein and parvulins (Rudd et al., 1995); both did not bind to or were not inhibited by CsA, which is specific for cyclophilins (
Trandinh et al., 1992).
The CsA has anti-parasitic effect that is well known however little has been known about its effect on
Giardia. In this study,
G. intestinalis was found to be susceptible to CsA in vitro, as most protozoan parasites were. The susceptibility of trophozoite PPIase to CsA correlated with the presence of essential tryptophan within the CsA binding site of Gicyp. A good correlation was observed between the concentration of CsA in the culture medium and the inhibition of trophozoite proliferation (data not shown). The 50% growth inhibits concentration (IC
50) of
E. histolytica,
Plasmodium vivax and
P. falciparum were 1.0, 1.7, and 0.12-0.5 µg/ml, respectively (
Reddy et al., 1995;
Kocken et al., 1996;
Ostoa-Saloma et al., 2000). Interestingly, some parasites were reported to be insensitive to CsA. IC
50 values of
Brugia malayi and
Toxoplasma gondii were calculated to be 860 and 32.5 nM, respectively (
Page et al., 1995). And,
Leishimania major was not affected by concentrations less than 25 µg/ml. The differences among species in susceptibility to CsA may simply be due to differential drug uptake or different residue in place of the conserved tryptophan residue, which is essential to bind to CsA (
Page et al., 1995).
The precise mechanism by which CsA exerts these anti-parasitic effects and the reason for the apparent species differences are yet to be elucidated. Nevertheless the mode of action may involve inhibition of the PPiase activity of endogenous cyclophilin or inhibition of an essential signal-transduction pathway, perhaps, via inhibition of a calcineurin homolog, which has been described for the immunosuppuression of T cells (
Clipstone and Crabtree, 1992).
References
- 1. Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev 2001;14:447-475.
- 2. Bergsma DJ, Eder C, Gross M, et al. The cyclophilin multigene family of peptidyl-prolyl isomerase. J Biol Chem 1991;266:23204-23214.
- 3. Chappell LH, Thomson AW. Studies on the action of cyclosporin A against Schstosoma mansoni and other parasitic infections. Transplant Proc 1988;20:291-297.
- 4. Clipstone NA, Crabtree GR. Identification of calcineurin as a key signaling enzyme in T-lymphocyte activation. Nature 1992;357:695-697.
- 5. Duina AA, Marsh JA, Kurtz RB, Chang HJ, Lindquist S, Gaber RF. The peptidyl-prolyl isomerase domain of the CyP-40 cyclophilin homology Cpr7 is not required to support growth or glucocorticoid receptor activity in Saccharomyces cerevisiae. J Biol Chem 1998;273:10819-10822.
- 6. Fischer G, Wittmann-Liebold B, Lang K, Keifhaber T, Schmid FX. Cyclophilin and peptidyl-prolyl cis trans isomerase are probably identical proteins. Nature 1989;337:476-478.
- 7. Gasser CS, Gunning DA, Budelier KA, Brown SM. Structure and expression of cytosolic cyclophilin/peptidyl-prolyl cis-trans isomerase of higher plants and production of active tomato cyclophilin in Escherichia coli. Proc Natl Acad Sci USA 1990;87:9519-9523.
- 8. Haendler B, Hofer-Warbinek R, Hofer E. Complementary DNA for human T-cell cyclophilin. EMBO J 1987;6:947-950.
- 9. Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 1984;226:544-547.
- 10. Kocken CH, van der Wel A, Rosenwirth B, Thomas AW. Plasmodium vivax: in vitro antiparasitic effect of cyclosporins. Exp Parasitol 1996;84:439-443.
- 11. Liu J, Walsh CT. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc Natl Acad Sci USA 1990;87:4028-4032.
- 12. Liu J, Chen CM, Walsh CT. Human and Escherichia coli cyclophilins: sensitivity to inhibition by the immunosuppressant cyclosporin A correlates with a specific tryptophan residue. Biochemistry 1991;30:2306-2310.
- 13. Minotto L, Tutticci EA, Bagnara AS, Schofield PJ, Edwards MR. Characterization and expression of the carbamate kinase gene from Giardia intestinalis. Mol Biochem Parasitol 1999;98:43-51.
- 14. Ostoa-Saloma P, Cesar Carrero J, Petrossian P, Herion P, Landa A, Pedro Laclette J. Cloning, characterization and functional expression of a cyclophilin of Entamoeba histolytica. Mol Biochem Parasitol 2000;107:219-225.
- 15. Page AP, Kumar S, Carlow CKS. Parasite cyclophilins and antiparasite activity of cyclosporin A. Parasitol Today 1995;11:385-389.
- 16. Reddy GR. Cloning and characterization of a Plasmodium falciparum cyclophilin gene that is stage-specifically expressed. Mol Biochem Parasitol 1995;73:111-121.
- 17. Schneider H, Charara N, Schmitz R, et al. Human cyclophilin C: Primary structure tissue distribution and determination of binding specificity for cyclosporins. Biochemistry 1994;33:8218-8224.
- 18. Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusion proteins with glutathione-S-transferase. Gene 1988;67:31-40.
- 19. Sogin ML, Hinkle G, Leipe DD. Universal tree of life. Nature 1993;362:795.
- 20. Solbach W, Forberg K, Rollinghoff M. Effect of T-lymphocyte suppression on the parasite burden in Leishmania major-infected, genetically susceptible BALB/c mice. Infect Immun 1986;54:909-912.
- 21. Spik G, Haendler B, Delmas O, et al. A novel secreted cyclophilin-like protein (SCYLP). J Biol Chem 1991;266:10735-10738.
- 22. Trandinh CC, Pao GM, Saier MH Jr. Structural and evolutionary relationships among the immunophilins: two ubiquitous families of peptidyl-prolyl cis-trans isomerases. FASEB J 1992;6:3410-3420.
- 23. Yee J, Mowatt MR, Dennis PP, Nash TE. Transcriptional analysis of the glutamate dehydrogenase gene in the primitive eukaryote, Giardia lamblia. Identification of a primordial gene promoter. J Biol Chem 2000;275:11432-11439.
Fig. 1Nucleotide sequences and putative amino acid sequences of Gicyp1. Putative transcriptional signal sequence 'tataa' is shown as boxed sequence. The bolded and underline sequences 'agtaaa' indicate polyadenylation consensus sequence. The italic characters of nucleotide sequences were detected by 5' RACE PCR analyses. The gray-boxed characters represent initiation signals in Giardia.

Fig. 2A comparison of the deduced amino acid sequences of Giardia cyclophilin with other cyclophilins. Bolded amino acid sequences indicate residues involved in the CsA binding. The tryptophan essential for CsA binding is indicated with an asterisk. G.int, G. intestinalis; E.his, Entamoeba histolytica (accession number AF017993); P.fal, Plasmodium falciparum (accession number U33869); B.mal, Brugia malayi (accession number U47811); L.maj, Leshimania major (accession number Y13576). Yeast, accession number X17505; mouse, accession number X52803; human, accession number X52851; rat, accession number M19533.

Fig. 3Southern hybridization (A) and Northern hybridization (B) of Giardia intestinalis cyclophilin gene. (A) The genomic DNA of G. intestinalis were digested with Pst I (Lane 1), Eco RI (Lane 2), and Hind III (Lane 3).
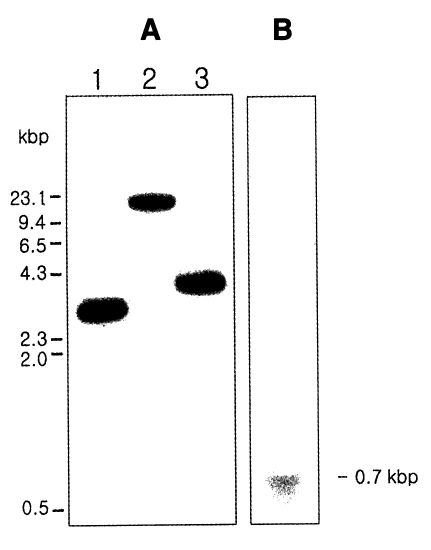
Fig. 4SDS-PAGE gel electrophoresis patterns of recombinant GST fusion protein and purified Gicyp protein. Lane 1, crude extract of recipient strain E. coli BL21; 2, crude extract of transformed BL21 expressing Gicyp; 3, purified GST-Gicyp fusion protein; 4, purified Gicyp.
Fig. 5
Giardia Gicyp is an active and cyclosporin A - sensitive PPIase in protease-coupled peptide assay. The time course of cis → trans isomerase of N-Suc-Ala-Ala-Pro-Phe-p-nitroanilide coupled with chymotryptic cleavage was measured by the increase of absorbance at 390 nm using Shimadzu UV-1201 spectrophotometer. The data were collected every 5 s over 3-8 min interval. The isomerization of the peptide substrate (c; negative control) was accelerated in the presence of Gicyp1 at 20 nM (a). This PPIase activity was blocked after incubation of 20 nM Gicyp with 0.5 µM CsA (b).