Abstract
Cryptosporidium and Giardia are 2 protozoan parasites responsible for waterborne diseases outbreaks worldwide. In order to assess the prevalence of these protozoans in drinking water samples in the northern part of Portugal and the risk of human infection, we have established a long term program aiming at pinpointing the sources of surface water, drinking water, and environmental contamination, working with the water-supply industry. Total 43 sources of drinking water samples were selected, and a total of 167 samples were analyzed using the Method 1623. Sensitivity assays regarding the genetic characterization by PCR and sequencing of the genes, 18S SSU rRNA, for Cryptosporidium spp. and β,-giardin for G. duodenalis were set in the laboratory. According to the defined criteria, molecular analysis was performed over 4 samples. Environmental stages of the protozoa were detected in 25.7% (43 out of 167) of the water samples, 8.4% (14 out of 167) with cysts of Giardia, 10.2% (17 out of 167) with oocysts of Cryptosporidium and 7.2% (12 out of 167) for both species. The mean concentrations were 0.1-12.7 oocysts of Cryptosporidium spp. per 10 L and 0.1-108.3 cysts of Giardia duodenalis per 10 L. Our results suggest that the efficiency in drinking water plants must be ameliorated in their efficiency in reducing the levels of contamination. We suggest the implementation of systematic monitoring programs for both protozoa. To authors' knowledge, this is the first report evaluating the concentration of environmental stages of Cryptosporidium and Giardia in drinking water samples in the northern part of Portugal.
-
Key words: Cryptosporidium spp., Giardia duodenalis, drinking water, risk assessment, public health, Portugal
INTRODUCTION
Cryptosporidium spp. and
Giardia duodenalis are 2 protozoan parasites that affect humans and a wide range of domestic and wild animals [
1-
3]. These parasites are a major cause of diarrheal disease in humans and animals worldwide, causing high morbidity in their hosts, and in immunocompromised hosts, they can lead to death.
The
Cryptosporidium genus comprises of more than 20 species, 12 of which are reported to infect mammals.
G. duodenalis is a species complex of the genus
Giardia, comprising of several genotypes or assemblages. The transmission of these 2 parasites is sustained by zoonotic (animal to human) and anthroponotic (human to human) cycles where several species and genotypes are enrolled [
3-
5]. Water is the major transmission route of
Cryptosporidium and
Giardia, where they can resist and remain infective due to their robust form, the oocyst and cyst, respectively [
6,
7]. The infected hosts shed in the environment a large number of these transmissive and infective stages, contributing to an increase of environmental contamination, in particular water courses. Cysts and oocysts not only remain infective for long periods in environment but are also resistant to the conventional treatment processes of water, representing a serious problem of public health [
2,
8-
12]. This problem is also potentiated by the fact that the number of parasites required to induce infection is small, i.e., infectious dose of 83-123 oocysts for
Cryptosporidium spp.and 19-50 cysts for
G. duodenalis [
1,
13]. Extrapolations from the data collected in the world largest
Cryptosporidium outbreak associated with water consumption, the Milwaukee outbreak, indicate an even lower infectious dose from 1 to 10 oocysts [
14].
The sources of drinking water include rivers, reservoirs, canals, or low land reservoirs. The pathogens can enter these surface waters from agricultural or urban runoff, wastewater treatment discharges or biosolids causing a cycle of infection to humans and animals [
7,
8,
15]. In many countries, such as Portugal, there are no requirements for testing surface waters for the presence of these parasites; it is now clear, even through the analysis of outbreaks, that the pathogens can pass into recreational and drinking water [
9,
10,
12].
The northern region of Portugal contains the highest density of bovine farms and also a high population density (176 inhabitants per km
2) of the country. Curiously, only 84% of the whole resident population has access to drinking water supply system. Significant concentrations of the infectious stages of both parasites have been found in water samples collected from rivers in the southern region of Portugal [
12,
16,
17]. Also, studies on human and animal biological reservoirs indicated an important presence of zoonotic species [
18-
22].
The specific aim of the present study was to evaluate the prevalence and levels of concentration of the environmental stages of Cryptosporidium spp. and G. duodenalis in drinking water samples from the network plants of the northern region of Portugal, assessing the risk of human infections associated with water consumption.
MATERIALS AND METHODS
The northern region of Portugal
In the north of Portugal there are 5 major hydrographical basins forming the most important water resources of the country. These hydrographical basins are named after the main rivers; Minho, Lima, Cávado, Ave, and Douro (
Figs. 1,
2). Cávado and Ave rivers run entirely inside national (Portuguese) borders, while Minho, Lima, and Douro are international rivers, with sources in Spain. Global features of the area, based on data provided by CCDRN, are as follows: The resident population in 2006 is 3,744,341 inhabitants; animal husbandry is an important economic activity; there are poorly developed sanitation infrastructures with wastewater plants discharging into these hydrographical basins; 64% of resident population has access to sanitation and 84% has access to treated drinking water; the region has the highest population density of the country, reaching 176 inhabitants per km
2, there are a high number of recreational areas, river beaches as well as pumping areas for drinking water plants.
Drinking water samples were collected twice a year from January 2004 to December 2006 in 43 sampling points in the drinking water treatment plants, from upriver to downriver, from all the 5 hydrographical basins (
Fig. 2). The volume of each sample ranged from 80 to 100 L. Samples were collected locally, filtered through Filta-Max filters (IDEXX Laboratories, Inc., Westbrook, Maine, USA) with a pump on the inlet side of the filter according to the recommendation of the manufacturer. Intact filters were kept in refrigerated containers and transported immediately to the laboratory. The filter was taken from the container and processed with the aid of a Filta-Max Manual Wash Station (IDEXX Laboratories, Inc.) for further elution and concentration process which consisted of decompression of the filter, passing the sample through a membrane, and centrifugation. A sample pellet (around 2 µl) was obtained and transferred to a Leighton tube for subsequent immunomagnetic separation (IMS).
The IMS procedure was performed according the US EPA method 1623 (USEPA, 2001). Briefly, anti-Giardia and anti-Cryptosporidium magnetic beads were mixed with SL Buffer A and SL Buffer B in each Leighton tube containing the sample concentrate (Dynabeads GC-Combo, Invitrogen Dynal, A. S., Oslo, Norway) and incubated for 1 hr at room temperature. Using 2 magnetic particle concentrators, beads were collected, washed, and transferred into a 1.5 µl tube. Then, 50 µl of 0.1 N HCl were added to each sample to dissociate beads from the target organisms, the beads were rejected and the suspension was transferred to the wells of the slides containing 5 µl of 1.0 N NaOH. The samples were air dried overnight and stained with FITC-conjugated anti-Cryptosporidium spp. and anti-Giardia spp. monoclonal antibodies, according the manufacturer's instructions (Crypto/Giardia Cells, Cellabs, Australia). The excess of FITC-MAb was removed by adding 100 µl of PBS to each well, leaving the slides for 5 min, and aspirating the excess of PBS. A 50-µl aliquot of 4'-6'-diamino-2-phenylindole (DAPI) solution (0.4 µg/ml in PBS) was introduced into each well. The slides were left at room temperature for 15 min, and excess DAPI solution removed by washing the slides in PBS. Slides were examined by epifluorescence microscope. Giardia cysts and Cryptosporidium oocysts were indentified and counted based on their shape and size using a Nikon Optiphot fluorescence microscope (Nikon Corporation, Tokyo, Japan). The number of cysts and oocysts per each well was recorded and concentrations extrapolated per 10 L of sample. Positive and negative controls were performed as indicated by the manufacturer and recommended in the Method 1623.
The mean recovery percentages of oocysts of
Cryptosporidium spp. and cysts of
G. duodenalis using Filta-Max system and IMS procedures from water samples were, according to the manufacturer, 50 ± 13% and 41 ± 79%, respectively [
23].
PCR analysis was performed in the samples with the highest density of infectious stages of both parasites detected by the direct fluorescence assay (DFA). The criterion utilized was the detection of a minimum of 100 cyst or oocyst stage of any parasite in the total sample volume. In this context, the genetic characterization was executed in 80 samples. The cover slip was separated from the slide, and with the aid of cotton swab soaked with 100 µl of distillated water, the surface of the slide was scraped in order to collect the sample. It was confirmed, under microscopic observations, that the slide had no remaining cysts or oocysts. The tip of the cotton swab was cut and placed in a 1.5 µl tube for subsequent DNA extraction with a QIAamp DNA Mini Kit (QIAGEN GmbH, Berlin, Germany), according to the manufacturer's instructions.
For determining the species of
Cryptosporidium and
Giardia present in the samples, a PCR analysis was performed. A 2-step nested PCR was performed to amplify a portion of the small-subunit (SSU) ribosomal RNA gene of
Cryptosporidium [
24]. For the molecular typing of
G. duodenalis, a semi-nested PCR was performed to amplify a portion of the β-giardin gene [
25]. For all PCR reactions, negative and positive controls were prepared, with sterile water and reference DNA, respectively. The PCR products were analyzed in agarose gel (1.4%) stained with ethidium bromide under UV light. Images were captured with a gel documentation system (GelDoc2000, BioRad, Hercules, California, USA).
RESULTS
IMS and DFA detection of infectious stages of Cryptosporidium and Giardia
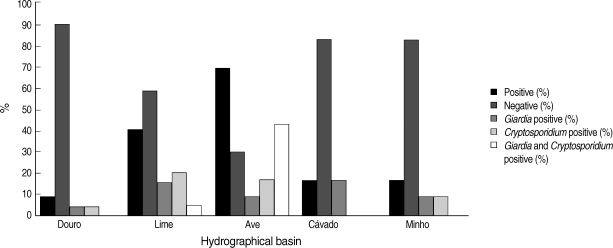
The number of validated drinking water samples in this study was 167. In
Fig. 3, the percentage and concentration of isolates from the 5 hydrographical basins are shown. Negative results, for both protozoa, were obtained in 124 out of 167 drinking water samples. Infectious stages of the protozoa were detected in 25.7% (43 out of 167) of the water samples. Among them, 8.4% (14 out of 167) of the samples were with cysts of
Giardia, 10.2% (17 out of 167) with oocysts of
Cryptosporidium, and 7.2% (12 out of 167) with both parasites. In positive water samples, no empty (i.e., without internal characteristics, or ghosts) or DAPI negative oocysts of
Cryptosporidium spp. or cysts of
G. duodenalis were found. Furthermore, the mean concentrations of
G. duodenalis cysts were much higher than those of the
Cryptosporidium spp. oocysts (0.1-108.3 cysts per 10 L and 0.1-12.7 oocysts per 10 L, respectively). A number of cysts and oocysts greater than to the minimal infectious dose (83-123 oocysts for
Cryptosporidium and 19-50 cysts for
Giardia) were detected in 2 samples. Except a third sample, where the amount of cysts of
Giardia was 12.6 cysts per 10 L, none of the samples had more than 5 cysts or oocysts per 10 L, showing a low level of contamination.
It was not possible by PCR to amplify DNA extracted from slides containing less than 50 oocysts of Cryptosporidium and 50 cysts of Giardia. Furthermore, positive amplifications over 3 replicates were never obtained with the number of cysts and oocysts smaller than 1,000 per slide. With this criterion, of all the positive IMS samples, PCR amplification was performed only over 4 samples. In these samples, it was not able to obtain amplification. In the correspondent raw water samples at the entrance of the drinking water plant, Cryptosporidium andersoni, Cryptosporidium parvum, Cryptosporidium hominis, and Cryptowsporidium muris have been detected (personal communication). Also, G. duodenalis assemblage A-II (in 4 samples), assemblage B (1 sample), and assemblages A, B, and E together (in 3 samples) was found in the same raw water (personal communication). It is expected that at the end of the treatment process the same species and genotypes will be found.
DISCUSSION
The results of a previous study indicated that the infectious stages of
Cryptosporidium spp. and
G. duodenalis are widely distributed in the rivers of northern Portugal in very significant concentrations (personal communication). In that work, it was found that 73% of a total number of 283 raw water samples were positive for environmental stages of
Cryptosporidium or
Giardia. Also, the levels of contamination were very high; a range of 0.17-50,000 cysts of
Giardia per 10 L and 0.2-726.1 oocysts of
Cryptosporidium per 10 L. Moreover, studies on human and animal biological reservoirs in the northern region of Portugal indicated an important presence of zoonotic species [
18-
21].
Surface waters (rivers, reservoirs, canals, and low land reservoirs) are used to produce drinking water for human consumption. However, no special treatment is applied to the water for animal consumption. The surface water collected from the rivers is used as drinking water for the animals or used for agricultural purposes, by the majority of farmers. The contamination of husbandry in these conditions is greater. In fact, has been shown in a previous study a prevalence of 25% of
Cryptosporidium infections in 467 bovine fecal samples in northern Portugal [
21]. Producers must be advertized about the risk of infection for both protozoa, and the low efficiency in animal production.
In the present study, it is observed that drinking water for human consumption is produced in very efficient plants regarding the elimination of these parasites. In the majority of samples, no environmental stages of
Cryptosporidium or
Giardia were observed. In the positive samples, the amounts were largely lower than the infectious dose [
1,
13,
14]. Although lack of efficiency was observed only in 1 plant, the levels of the environmental stages were above the infectious dose determined for these protozoa. In fact, the concentrations of both protozoa found in the respective collection point of raw water of this water treatment plant were similar to the ones observed in treated water, although the time of sampling was not the same. Moreover, the 3 samples presenting levels of contamination above 5 cysts or oocysts per 10 L were collected in the same water treatment plant.
Molecular typing was unsuccessful on these water samples. Sensitivity assays were set in the laboratory for the PCR reactions, and the number of 50 cysts or oocysts was our limit of sensitivity. Due to this fact and taking into account the low levels of contamination in the samples obtained in this study, the majority of the samples were out of selection for PCR amplification. The remaining samples, in which the number of cysts or oocysts was higher than this sensitivity limit, were subjected to DNA extraction and PCR, although the amplification did not occur. In the sensitivity assays set in the laboratory, it is not observed reproducibility in the PCR when the number of cysts or oocysts used in the slide ranged from 50 to 1,000 oocysts or cysts. The parasite load in the samples subjected to DNA extraction and PCR was in all cases much less than 1,000 oocysts or cysts. To our knowledge, there are no set protocols to perform DNA extraction and PCR over these protozoa from the slides. Moreover, even if IMS is applied in the isolation of the parasite, the final samples are not always free of contamination or inhibitors; therefore, negative PCRs may also be due to this fact [
26].
The results of this study seem to indicate that the risk assessment for cryptosporidiosis and giardiasis for humans is low in the north of Portugal. However, for this, the condition that whole population has access to the network system for drinking water needs to be fulfilled, and, in this case, this condition is not observed for a very significant segment of the northern population. First of all, according to the national statistics, only 84% of the population has access to the drinking water system; second, among the population covered by the drinking water distribution systems (84%), a significant segment are provided by water obtained in wells or other origin. Old habits, water prices, and lack of information about the quality of the treated water are the main reasons referred. Taking into account these aspects and the real number of people consuming water for the drinking water systems supply, it is expected that one third of the northern Portuguese population is exposed to the infection by Cryptosporidium and Giardia due to consumption of water.
Portuguese scientific programs take into account the European strategic plan for Animal Health (2007-2013), "prevention is better than cure". Thus, research is directed to increase competitiveness of the livestock production systems, to improve standards of animal health and welfare, and to prevent and control animal pathologies with particular emphasis on emerging diseases and zoonosis. The assessment of biological contamination of the environment and superficial waters, as a pre-harvest requirement, has been an increasingly important area of research.
Drinking water produced in the north of Portugal presents very good standards of microbiological quality, concerning these 2 protozoa. Inefficient water treatment plants were identified and a rebuilt program was implemented. Also, after the correction of the identified failures in the process of drinking water production guided by Portuguese water-supply companies, a great priority needs to be implemented; the guaranty of access of whole population to the drinking water-supply system.
ACKNOWLEDGEMENTS
The authors are grateful to water-supply industry from the northern region of Portugal; Águas do Douro e Paiva, Águas do Cávado, Águas do Ave, Águas do Minho e Lima, Águas de Trás-os-Montes e Alto Douro, Serviços Municipalizados de Viana de Castelo, e Vimágua. Authors are grateful to Dr. António Rocha for the revision of the manuscript. This work was financially supported by funds of Project 61018, Action Environment and Health, from "Fundação Calouste Gulbenkian". André Almeida is supported by a grant of Portuguese "Fundação para a Ciência e a Tecnologia" number SFRH/BD/22763/2005.
References
- 1. Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev 2001;14:447-475.
- 2. Fayer R. Cryptosporidium: a water-borne zoonotic parasite. Vet Parasitol 2004;126:37-56.
- 3. Fayer R. Taxonomy and species delimitation in Cryptosporidium. Exp Parasitol 2009;3. 18.
- 4. Xiao L, Fayer R. Molecular characterisation of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int J Parasitol 2008;38:1239-1255.
- 5. Caccio SM, Ryan U. Molecular epidemiology of giardiasis. Mol Biochem Parasitol 2008;160:75-80.
- 6. Thompson RC. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int J Parasitol 2000;30:1259-1267.
- 7. Fayer R, Morgan U, Upton SJ. Epidemiology of Cryptosporidium: transmission, detection and identification. Int J Parasitol 2000;30:1305-1322.
- 8. Caccio SM, De Giacomo M, Aulicino FA, Pozio E. Giardia cysts in wastewater treatment plants in Italy. Appl Environ Microbiol 2003;69:3393-3398.
- 9. Castro-Hermida JA, Garcia-Presedo I, Almeida A, Gonzalez-Warleta M, Correia Da Costa JM, Mezo M. Contribution of treated wastewater to the contamination of recreational river areas with Cryptosporidium spp. and Giardia duodenalis. Water Res 2008;42:3528-3538.
- 10. Castro-Hermida JA, Garcia-Presedo I, Almeida A, Gonzalez-Warleta M, Correia Da Costa JM, Mezo M. Presence of Cryptosporidium spp. and Giardia duodenalis through drinking water. Sci Total Environ 2008;405:45-53.
- 11. Castro-Hermida JA, Garcia-Presedo I, Almeida A, Gonzalez-Warleta M, Correia Da Costa JM, Mezo M. Detection of Cryptosporidium spp. and Giardia duodenalis in surface water: a health risk for humans and animals. Water Res 2009;43:4133-4142.
- 12. Lobo ML, Xiao L, Antunes F, Matos O. Occurrence of Cryptosporidium and Giardia genotypes and subtypes in raw and treated water in Portugal. Lett Appl Microbiol 2009;48:732-737.
- 13. Chappell CL, Okhuysen PC, Langer-Curry R, Widmer G, Akiyoshi DE, Tanriverdi S, et al. Cryptosporidium hominis: experimental challenge of healthy adults. Am J Trop Med Hyg 2006;75:851-857.
- 14. Dillingham RA, Lima AA, Guerrant RL. Cryptosporidiosis: epidemiology and impact. Microbes Infect 2002;4:1059-1066.
- 15. Hunter PR, Thompson RC. The zoonotic transmission of Giardia and Cryptosporidium. Int J Parasitol 2005;35:1181-1190.
- 16. Alves M, Ribeiro AM, Neto C, Ferreira E, Benoliel MJ, Antunes F, et al. Distribution of Cryptosporidium species and subtypes in water samples in Portugal: a preliminary study. J Eukaryot Microbiol 2006;53(Suppl 1):S24-S25.
- 17. Alves M, Xiao L, Antunes F, Matos O. Distribution of Cryptosporidium subtypes in humans and domestic and wild ruminants in Portugal. Parasitol Res 2006;99:287-292.
- 18. Almeida AA, Delgado ML, Soares SC, Castro AO, Moreira MJ, Mendonca CM, et al. Genotype analysis of Giardia isolated from asymptomatic children in northern Portugal. J Eukaryot Microbiol 2006;53(Suppl 1):S177-S178.
- 19. Almeida AA, Delgado ML, Soares SC, Castro AO, Moreira MJ, Mendonca CM, et al. Genetic characterization of Cryptosporidium isolates from humans in northern Portugal. J Eukaryot Microbiol 2006;53(Suppl 1):S26-S27.
- 20. Matos O, Alves M, Xiao L, Cama V, Antunes F. Cryptosporidium felis and C. meleagridis in persons with HIV, Portugal. Emerg Infect Dis 2004;10:2256-2257.
- 21. Mendonca C, Almeida A, Castro A, de Lurdes Delgado M, Soares S, da Costa JM, et al. Molecular characterization of Cryptosporidium and Giardia isolates from cattle from Portugal. Vet Parasitol 2007;147:47-50.
- 22. Sousa MC, Morais JB, Machado JE, Poiares-da-Silva J. Genotyping of Giardia lamblia human isolates from Portugal by PCR-RFLP and sequencing. J Eukaryot Microbiol 2006;53(Suppl 1):S174-S176.
- 23. McCuin RM, Clancy JL. Modifications to United States Environmental Protection Agency methods 1622 and 1623 for detection of Cryptosporidium oocysts and Giardia cysts in water. Appl Environ Microbiol 2003;69:267-274.
- 24. Xiao L, Morgan UM, Limor J, Escalante A, Arrowood M, Shulaw W, et al. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl Environ Microbiol 1999;65:3386-3391.
- 25. Caccio SM, De Giacomo M, Pozio E. Sequence analysis of the beta-giardin gene and development of a polymerase chain reaction-restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int J Parasitol 2002;32:1023-1030.
- 26. Jiang J, Alderisio KA, Singh A, Xiao L. Development of procedures for direct extraction of Cryptosporidium DNA from water concentrates and for relief of PCR inhibitors. Appl Environ Microbiol 2005;71:1135-1141.
Fig. 1Geographic location of the North of Portugal and its 5 hydrographical basins into Iberian Peninsula.
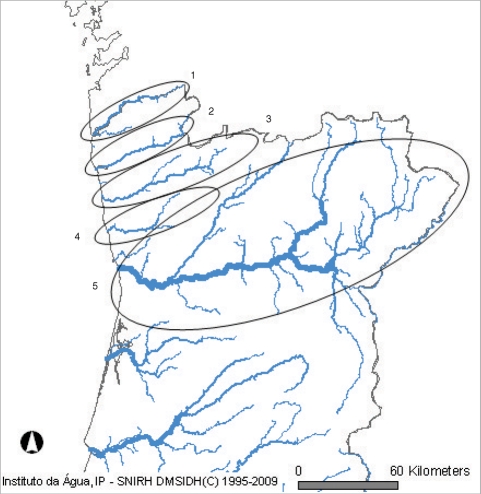
Fig. 2Location of the 5 hydrographical basins in the North of Portugal. 1. Minho; 2. Lima; 3. Cávado; 4. Ave; 5. Douro.
Fig. 3Distribution of the results obtained by Method 1623 EPA-USA for infectious stages of Cryptosporidium spp. and Giardia duodenalis in drinking water samples collected in the 5 hydrographical basins.