Abstract
Rats were immunized through an initial infection with 1,000 filariform larvae (L3) of Nippostrongylus brasiliensis and after complete expulsion of worms they were challenged with 1,000 L3 of Strongyloides venezuelensis to investigate whether cross-resistance developed against a heterologous parasite. Nippostrongylus brasiliensis-immunized rats developed a partial cross-resistance against S. venezuelensis migrating larvae (MSL3) in the lungs and adult worms in the small intestine. The population of MSL3 in the lungs were significantly lower (P<0.05) in immunized rats (22.0 ± 7.4) compared with controls (105.0 ± 27.6). The populations of adult worms, egg output and fecundity were initially decreased but from day 14 post-challenge they did not show any significant difference between immunized and control rats. However, the length of worm in immunized rat was revealed as retardation. Peripheral blood eosinophilia was significantly decreased (P<0.05) on day 7 post-challenge and then gradually increased, which peaked on day 42 post-challenge when most of the worms were expelled. These results suggest that peripheral blood eosinophilia is strongly involved in the worm establishment and expulsion mechanisms.
-
Key words: Strongyloides venezuelensis, Nippostrongylus brasiliensis, filariform larvae, cross-resistance, fecundity, peripheral blood eosinophilia
INTRODUCTION
Analysis of cross-resistance between heterologous and/or homologous parasites has been considered important for better understanding of immuno-ecological aspects of host-parasite relationships.
Strongyloides venezuelensis, a parasitic nematode of rats, has recently been used as a model for studying the host-parasite relationship of human and/or animal strongyloidiasis (
Sato and Toma, 1990a;
Taira et al., 1994). This parasite enters into the host through skin penetration and then migrates to the lungs, trachea, and reaches to the small intestine, where it moults to become a sexually mature adult. Natural mixed infections of
S. venezuelensis and
S. ratti, another closely related species of the genus
Strongyloides, in wild rats have been reported previously (
Little, 1961;
Wertheim and Lengy, 1964). It has also been observed that mice immunized with
S. ratti developed cross-resistance to the intestinal stage but not to the migrating stage of
S. venezuelensis (
Korenaga et al., 1995).
Nippostrongylus brasiliensis, an intestinal nematode of rat, which has also been extensively used as a model to investigate immune responses to helminths in the murine hosts (
Ogilvie and Jones, 1971;
Sinski and Holmes, 1977;
Stadnyk et al., 1990). This strongylid, like
S. venezuelensis, enters into host through skin penetration, migrates to the lungs where it moults to become 4th-stage larva, and then travels to the small intestine where it takes a final moult to become a mature adult (
Bohn and Konig, 1985). It was reported that
N. brasiliensis-immunized rats showed marked reduced worm burdens and egg outputs after a challenge infection with
S. ratti (
Kazacos and Thorson, 1975;
Nawa et al., 1982). Natural concurrent infections with
N. brasiliensis and
S. venezuelensis in rats, have not been reported so far. However, Horii et al. (
1993) observed that
S. venezuelensis persisted for over 10 weeks, while
N. brasiliensis was expelled by 3 weeks without having interfered with each other in Mongolian gerbils.
In the present study, we have demonstrated that rats immunized through an initial infection with 1,000 filariform larvae (L3) of N. brasiliensis developed partial cross-resistance against migrating stage larvae (MSL3) in the lungs and adult worms in the small intestine as judged by body length, egg outputs and worm fecundity of a heterologous parasite, S. venezuelensis. Peripheral blood eosinophilia (PBE) was also observed in N. brasiliensis-immunized rats.
MATERIALS AND METHODS
Animal and parasite
Inbred 8-week-old male Fischer (F344) rats with an average body weight of 150 g were used in this study. The L
3 of
S. venezuelensis and
N. brasiliensis were obtained from 0.12% nutrient broth cultures (
Baek et al., 1998a). In case of
N. brasiliensis, larvae were harvested from the cultures on day 7 post-cultivation. The L
3 were washed three times in sterile saline (SS) containing 200 units/ml penicillin and 200 units/ml streptomycin sulphate (Sigma, St. Louis, USA), and 1,000 L
3 concentrated in 250 µl SS were used as an infective dose for each rat.
On day 0, rats were immunized through an initial infection with 1,000 L3 of N. brasiliensis. After complete expulsion of adult worms at day 28 post-infection (PI) from these rats, they were challenged with 1,000 L3 of S. venezuelensis on day 28 PI, while age matched rats were served as control (non-infected rat). In order to determine the day of challenge infection with S. venezuelensis, normal pattern of egg outputs and worm expulsion were monitored in 20 rats infected with 1,000 L3 of N. brasiliensis on day 3, 7, 14 and 21 PI.
Recovery of worms
Worms were recovered at various intervals (e.g., day 3, 7, 14, 21, 28, 35, 42 and 49 post-challenge) from both immunized and control rats (
Islam et al., 1999). Briefly, the MSL
3 were collected from the lungs on day 3 post-challenge (PC). The lungs were minced into small pieces and incubated in SS at 37℃ for 4 hr in a Baermann device. The MSL
3 settled down at the bottom were counted under a dissecting microscope.
For counting the adult worms, the entire small intestine of rats sacrificed at various intervals was dissected out, opened longitudinally and incubated at 37℃ for 4 hr and worms were counted as mentioned above. The whole length of the small intestine was examined carefully under a dissecting microscope and worms attached to the mucosa were also counted.
Fecundity and fecal egg output of worms
The parasitic females developed after a challenge infection in
N. brasiliensis-immunized and control rats examined under a light microscope at various intervals to measure the fecundity in terms of eggs in uterus per worm (
Baek et al., 1998b).
Eggs per gram of feces (EPG) were determined daily by collecting fresh feces from five rats per group according to the method described previously (
Islam et al., 1999).
Morphological dimensions of the MSL3 recovered from the lungs on day 3 PC and the adult worms from the small intestines on day 7 PC of N. brasiliensis-immunized and control rats were examined. Fifty MSL3 were measured for their body length, esophagus, tail length, and body width at the esophago-intestinal (E-I) junction and at the anus. Fifty adult worms were measured for their body length, esophagus, tail length, and the length from the mouth to the vulva. Body width at the E-I junction, vulva and anus were also measured.
Peripheral blood eosinophilia
The number of eosinophils in peripheral blood was examined according to the method of Korenaga et al. (
1995). Briefly, five µl of the blood were taken from the tail vein and mixed with 20 µl of Hinkelman's solution (0.5% w/v eosin, 0.5% w/v phenol and 0.185% v/v formaldehyde in distilled water). Eosinophils were counted using an improved Neubauer haemocytometer.
Data obtained from immunized and control animals were analyzed by Student's t-test. Significance was determined at 5% level. Graphs were prepared by CA-Cricket Graph III.
RESULTS
Course of N. brasiliensis infection in rats
Following an initial infection with 1,000 L
3 of
N. brasiliensis in rats, eggs appeared first in the feces on day 6 PI, peaked on day 8 PI, and then declined sharply to zero by day 16 PI (
Table 1). The highest populations of adult worms were observed on day 7 PI, which were completely expelled by day 21 PI (
Table 1).
The number of
S. venezuelensis MSL
3 recovered from the lungs on day 3 PC was significantly lower (P<0.05) in
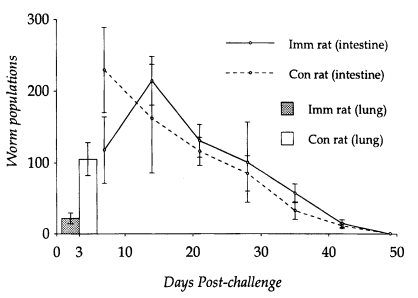
N. brasiliensis-immunized rats (22.0 ± 7.4) than in controls (105.0 ± 27.6). The highest number of adult worms was observed from the small intestine on day 14 PC (214.0 ± 33.9), while in controls, adult populations peaked on day 7 PC (229.4 ± 59.7) (
Fig. 1).
However, worm populations from day 14 PC onwards did not differ significantly between
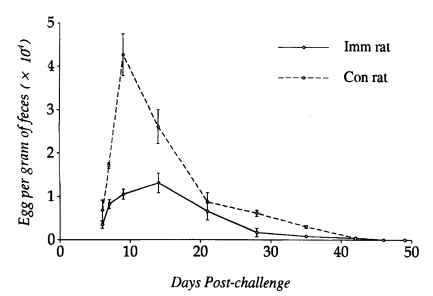
N. brasiliensis-immunized and control rats (P<0.05), and worms were completely expelled by day 49 PC. Eggs appeared in the feces on day 6 PC in both immunized and control rats. However, EPG counts peaked on day 12 PC in immunized rats and day 9 PC in controls, which were significantly depressed in the former group of rats at the initial stage of infection (P<0.05). From day 14 PC, EPG counts did not show significant difference in their kinetics, which gradually declined and reached zero by day 46 PC in both immunized and control rats (
Fig. 2).
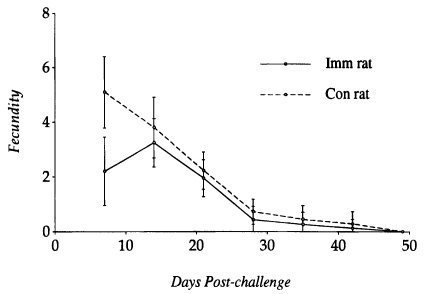
The fecundity of
S. venezuelensis parasitic females recovered from
N. brasiliensis-immunized rats and controls at various intervals was compared to each other (
Fig. 3). It peaked on day 14 PC in immunized rats and day 7 PC in controls. The fecundity of worms recovered on day 7 PC from immunized rats was significantly lower than those from controls (P<0.05), but on day 14 PC the worm fecundity did not show any significant difference between the two groups. Only a few worms contained eggs in their uteri at the time of expulsion from the small intestine of both immunized and control rats.
Morphological dimensions of MSL
3 recovered from the lungs on day 3 PC and adult worms from the small intestines on day 7 PC of immunized rats were compared with those from controls to determine whether the cross-resistance affects the morphological development of worms of heterologous challenge infection with
S. venezuelensis (
Table 2). The body length of both MSL
3 and adults recovered from
N. brasiliensis-immunized rats was significantly smaller than those from controls (P<0.05).
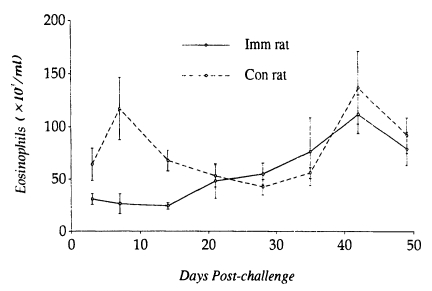
A significant relationship between low levels of PBE (24.1 ± 3.3×10
3/ml) and worm establishment and high levels of PBE (112.0 ± 18.4×10
3/ml) and worm expulsion in
N. brasiliensis-immunized rats. In contrast, PBE in controls tended to elevate (116.9 ± 29.4×10
3/ml) at early stages of infection (i.e., when worms are undergoing establishment in the small intestine), and then declined for a while, which in the late stage of infection again elevated to high levels (136.9 ± 34.2×10
3/ml) when most of the worms were expelled from the small intestine (
Fig. 4).
DISCUSSION
In the present investigation, we have demonstrated that rats after an initial infection with
N. brasiliensis acquired partial cross-resistance against a heterologous challenge infection with
S. venezuelensis. In this combination of parasites, it has been observed that cross-resistance was expressed against the population and morphological development of MSL
3 in the lungs, and also migration of these larvae to the small intestine. These findings partially confirm to the report of Nawa et al. (
1982) who observed that
N. brasiliensis-immunized rats developed cross-resistance against the tissue migrating stage, but not against the adult stage of challenged
S. ratti, a very closely related species to
S. venezuelensis.
In this study, the effect of partial cross-resistance was expressed against the adult worms, in particular, in body length of worms, fecundity and egg outputs at the initial stage of infection, but not expressed at the advanced stage (i.e., from day 14 PC onwards). Although the period for a peak worm population was delayed in
N. brasiliensis-immunized rats compared to controls, the worm population from day 14 PC did not show any significant difference in their kinetics, and worms were expelled by day 49 PC. The pattern of the worm expulsion and course of infection also did not differ between
N. brasiliensis-immunized and control rats. The present results suggest that host's acquired immunity to
N. brasiliensis could affect the migrating capability, morphological development and suppress reproduction of the worms of a heterologous challenge infection with
S. venezuelensis. However, it can not prevent the establishment of infection nor enhance the expulsion of adult worms in rats, because the effector/regulator cells involved in the worm expulsion are different and highly selective depending on the genus of intestinal helminths (
Horii et al., 1993;
Nawa et al., 1994). They described that the effector cells specific for
S. venezuelensis are mucosal mast cells which undergo hyperplasia in response to parasite specific antibodies, whereas goblet cells are considered specific for
N. brasiliensis.
Eosinophilia has long been known as a common clinical conditions of many parasitic infections and allergic inflammation (
Kay, 1979;
Wardlaw et al., 1995). High levels of peripheral and tissue eosinophilia have also been demonstrated in infections with other species of the genus
Strongyloides (
Katz, 1987;
Rotman et al. 1996). The importance of eosinophils in the protective immunity against
N. brasiliensis infection in mice has been demonstrated (
Shin et al., 1997). Korenaga et al. (
1991) reported that host's protective immunity against the tissue-migrating stage of
S. venezuelensis was anti-interleukin-5 (anti-IL-5) dependent, and that treatment with anti-IL-5 or anti-IL-5 receptor monoclonal antibody suppressed peripheral and tissue eosinophilia. However, the role of peripheral and tissue eosinophilia in the establishment and expulsion of gastrointestinal nematodes including
S. venezuelensis is still unclear (
Korenaga et al., 1995).
In the present study, we have demonstrated that establishment of heterologous challenge infection with
S. venezuelensis in
N. brasiliensis-immunized rats was correlated with low levels of peripheral eosinophilia, while a reverse phenomenon was shown in controls. Although high levels of peripheral eosinophilia has been observed in
S. venezuelensis-infected mice (
Korenaga et al., 1991), low levels of peripheral eosinophilia which favored the establishment of a heterologous challenge infection with
S. venezuelensis in
N. brasiliensis-immunized rats has not been reported previously. These results indicate that immuno-ecological aspect of host-parasite interaction in a cross-immune animal may provide us informations useful for understanding the establishment mechanisms of intestinal worms.
It has been reported that immunity to
S. venezuelensis infection is T-cell dependent (
Sato and Toma, 1990b). It may be possible that excretory-secretory antigens of the challenged worms suppress the synthesis and/or function of IL-5 secreted by T-cell populations, which generates eosinophil production (
Coffman et al., 1989;
Rennick et al., 1990), and thus facilitate worm establishment. Further studies are required to clarify this aspect.
Mechanisms by which gastrointestinal nematodes establish themselves in the predilection site have not been fully understood. Recently, however, Maruyama and Nawa (
1997) reported that
S. venezuelensis secrete a mucinous adhesive substances containing antigenic components, which was considered as a key step for the parasite to invade and establish in the host epithelial layer. Interestingly, in the late stage of infection, peripheral eosinophilia increased and reached to the peak level followed by complete expulsion of worms in both immunized and control rats. These findings further suggest that the relationship between eosinophilia and worm populations is immunologically mediated and eosinophils may act as an effector cell in the host protective immunity to
S. venezuelensis infection.
The present study may lead to the conclusion that host's protective immunity to a particular gastro-intestinal nematode may affect the morphological development and reproduction of worms of heterologous challenge infection, but could not express against worm establishment and expulsion mechanisms. PBE is strongly involved in worm establishment and expulsion mechanisms.
Notes
-
This research work was supported in part by the Biosafety Research Institute, Chonbuk National University (1998)
References
- 1. Baek BK, Islam MK, Kim JH. Development of an in vitro culture method for harvesting the free-living infective larvae of Strongyloides venezuelensis. Korean J Parasitol 1998a;36:15-22.
- 2. Baek BK, Islam MK, Matsuda K. Viability of eggs, filariform larvae and adults of Strongyloides venezuelensis (Nematoda: Strongyloidea) maintained in vitro. Korean J Parasitol 1998b;36:99-107.
- 3. Bohn A, Konig W. Characterization of antigens of the nematode Nippostrongylus brasiliensis by monoclonal antibodies. Z Parasitenkd 1985;71:663-672.
- 4. Brumpt E. Precis de Parasitologie. 1934, 6th ed. Paris, France. Masson et Cie.; p 1042.
- 5. Coffman RL, Seymour BWP, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science 1989;245:308-310.
- 6. Horii Y, Khan AI, Nawa Y. Persistent infection of Strongyloides venezuelensis and normal expulsion of Nippostrongylus brasiliensis in Mongolian gerbils, Meriones unguiculatus, with reference to the cellular responses in the intestinal mucosa. Parasite Immunol 1993;15:175-179.
- 7. Islam MK, Matsuda K, Kim JH, Baek BK. Effects of in vitro culture methods on morphological development and infectivity of Strongyloides venezuelensis filariform larvae. Korean J Parasitol 1999;37:13-19.
- 8. Katz FF. In Soulsby EJL ed, Immunity to Strongyloides. Immune responses in parasitic infections: Immunology, Immunopathology and Immunoprophylaxis. 1987, Vol I: Nematode:Florida, USA. CRC Press, Inc.; pp 89-125.
- 9. Kay AB. The role of the eosinophil. J Allergy Clin Immunol 1979;64:90-104.
- 10. Kazacos KR, Thorson RE. Cross-resistance between Nippostrongylus brasiliensis and Strongyloides ratti in rats. J Parasitol 1975;61:525-529.
- 11. Korenaga M, Hitoshi Y, Takatsu K, Tada I. Cross-resistance between Strongyloides venezuelensis and S. ratti in mice. J Helminthol 1995;69:119-123.
- 12. Korenaga M, Hitoshi Y, Yamaguchi N, Sato Y, Takatsu K, Tada I. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology 1991;72:502-507.
- 13. Little MD. Concurrent infections of Strongyloides venezuelensis and S. ratti in wild rats in New Orleans. J Parasitol 1961;47:17.
- 14. Maruyama H, Nawa Y. Strongyloides venezuelensis: Adhesion of adult worms to culture vessels by orally secreted mucous substance. Exp Parasitol 1997;85:10-15.
- 15. Nawa Y, Ishikawa N, Tsuchiya K, et al. Selective effector mechanisms for the expulsion of intestinal helminths. Parasite Immunol 1994;16:333-338.
- 16. Nawa Y, Mimori T, Korenaga M, Tada I. Stage-specific cross-resistance between Nippostrongylus brasiliensis and Strongyloides ratti (Nematoda) in rats. J Parasitol 1982;68:804-808.
- 17. Ogilvie BM, Jones VE. Nippostrongylus brasiliensis: a review of immunity and the host/parasite relationship in the rat. Exp Parasitol 1971;29:138-177.
- 18. Rennick DM, Thompson-Snipes L, Coffman RL, Seymour BWP, Jackson JD, Hudak S. In vivo administration of antibody to interleukin-5 inhibits increased generation of eosinophils and their progenitors in bonemarrow of parasitized mice. Blood 1990;76:312-316.
- 19. Rotman HL, Yutanawiboonchai W, Brigandi RA, et al. Strongyloides stercoralis: Eosinophil-dependent immune mediated killing of third stage larvae in BALB/cByJ mice. Exp Parasitol 1996;82:267-278.
- 20. Sato Y, Toma H. Strongyloides venezuelensis infections in mice. Int J Parasitol 1990a;20:57-62.
- 21. Sato Y, Toma H. Effects of spleen cells and serum on transfer of immunity to Strongyloides venezuelensis infection in hypothymic (nude) mice. Int J Parasitol 1990b;20:63-67.
- 22. Shin EH, Osada Y, Chai JY, Matsumoto N, Takatsu K, Kojima S. Protective roles of eosinophils in Nippostrongylus brasiliensis infection. Int Arch Allergy Immunol 1997;114:45-50.
- 23. Sinski E, Holmes PH. Nippostrongylus brasiliensis: Systemic and local IgG and IgA immunoglobulin responses in parasitized rats. Exp Parasitol 1977;43:382-389.
- 24. Stadnyk AW, McElroy PJ, Gauldie J, Befus AD. Characterization of Nippostrongylus brasiliensis infection in different strains of mice. J Parasitol 1990;76:377-382.
- 25. Taira N, Hirooka M, Saeki H. Isolation of Strongyloides venezuelensis from Rattus norvegicus in Kagoshima Prefecture. J Vet Med Sci 1994;56:255-258.
- 26. Wardlaw AJ, Moqbel R, Kay AB. Eosinophils: Biology and role in disease. Adv Immunol 1995;60:151-266.
- 27. Wertheim G. Experimental concurrent infections with Strongyloides ratti and S. venezuelensis in laboratory rats. Parasitology 1970;61:389-395.
- 28. Wertheim G, Lengy J. The seasonal occurrence of Strongyloides ratti (Sandground, 1925) and S. venezuelensis (Brumpt, 1934) in a population of Rattus norvegicus. J Helminthol 1964;38:393-398.
Fig. 1Kinetics of worm populations after a heterologous challenge infection with 1,000 L3 of Strongyloides venezuelensis in Nippostrongylus brasiliensis-immunized rats and controls was observed from the lung. Each value represents the mean ± SD of 5 rats.

Fig. 2Kinetics of fecal egg outputs after a heterologous challenge infection with 1,000 L3 of Strongyloides venezuelensis in Nippostrongylus brasiliensis-immunized rats and controls. Each value represents the mean ± SD of 5 rats.
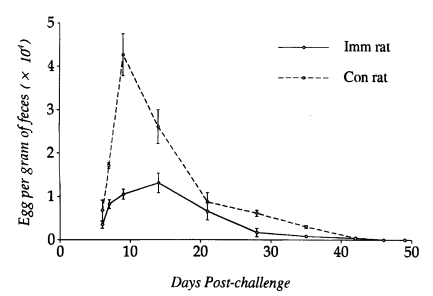
Fig. 3Kinetics of fecundity of the Strongyloides venezuelensis parasitic females recovered at various times from Nippostrongylus brasiliensisimmunized rats and controls after a challenge infection with 1,000 L3 of S. venezuelensis. Each value represents the mean ± SD of 50 worms.
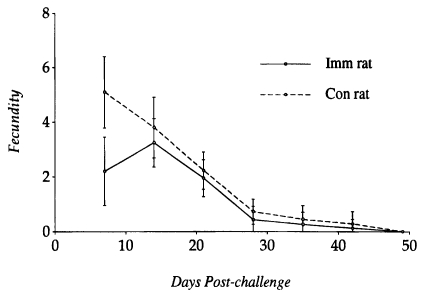
Fig. 4Kinetics of peripheral blood eosinophilia in Nippostrongylus brasiliensis-immunized rats and controls after a challenge infection with 1,000 L3 of Strongyloides venezuelensis. Each value represents the mean ± SD of 5 rats.
Table 1.Eggs per gram of feces (EPG) and number of adult worms recovered from rats infected with 1,000 L
3 of
Nippostrongylus brasiliensisa)
Table 1.
|
Day post-infection (DPI) |
Fecal egg output (EPG) |
Worm population |
|
3 |
NDb)
|
366.9 ± 89.9c)
|
|
6 |
1,840 ± 770 |
ND |
|
8 |
27,440 ± 5,609 |
489.8 ± 80.9d)
|
|
10 |
12,040 ± 3,634 |
ND |
|
12 |
3,600 ± 930 |
ND |
|
14 |
700 ± 469 |
21.4 ± 8.4d)
|
|
16 |
0 |
ND |
|
18 |
0 |
ND |
|
21 |
0 |
0 |
Table 2.Comparison of morphological dimensions of 50 migrating larvae (MSL
3) and 50 adult worms of
Nippostrongylus brasiliensis-immunized and control rats on day 3 after a challenge infection with 1,000 L
3 of
Strongyloides venezuelensisa)
Table 2.
|
Morphological dimensions (in μm) |
Worms recovered from
|
Immunized rats
|
Control rats
|
|
MSL3
|
Adult |
MSL3
|
Adult |
|
Length: |
|
|
|
|
|
Body |
433.7 ± 31.4b)c)
|
2329.0 ± 223.4b)
|
598.9 ± 45.6 |
2723.5 ± 168.7 |
|
Esophagus |
234.4 ± 25.5b)c)
|
612.4 ± 38.2 |
297.2 ± 21.2 |
618.1 ± 51.9 |
|
Tail |
49.0 ± 4.8b)c)
|
48.9 ± 3.4 |
71.4 ± 6.8 |
50.8 ± 3.2 |
|
Mouth to vulva |
|
1577.2 ± 121.6 |
|
1688.8 ± 234.3 |
|
Width at: |
|
|
|
|
|
E-I junctiona)
|
18.4 ± 1.0 |
32.2 ± 2.3 |
19.2 ± 1.1 |
32.9 ± 1.6 |
|
Anus |
13.0 ± 0.8 |
19.9 ± 1.9 |
13.8 ± 1.1 |
20.1 ± 1.4 |
|
Vulva |
|
41.0 ± 1.9 |
|
42.1 ± 1.4 |