Abstract
A cDNA expression library of Clonorchis sinensis adult worm was constructed, and screened out immunologically. One clone, pBCs31, was selected in view of its predominant reactivity with an experimentally infected rabbit serum. Recombinant C. sinensis antigen with 28 kDa as a β-galactosidase fusion protein produced in Escherichia coli was identified by immunoblot analysis. The cloned gene was composed of 16 copies of a 30 base pair repeat and an additional 320 bases. The deduced amino acid sequence of the tandem repeat was AQPPKSGDGG. On RNA slot blot analysis. C. sinensis adult worm RNA showed a positive reaction with the cloned gene. Enzyme-linked immunosorbent assay using a purified recombinant antigen of pBCs31 showed high specificity for diagnosis of clonorchiasis.
-
Key words: Clonorchis sinensis, immunodiagnosis, recombinant antigen, repetitive sequence
INTRODUCTION
Clonorchis sinensis is an important parasite of humans in Eastern Asia, including China, Taiwan, northern Vietnam and Korea. In Korea, it is well known that human infection of
C. sinensis is widely distributed along the great rivers and streams (
Rim, 1986).
Although stool examination is a definitive diagnostic method for clonorchiasis, continuous efforts have been made to improve the effectiveness of immunodiagnostic tests as supportive diagnostic methods for last few decades. For example, the intradermal test based on the immediate type hypersensitivity reaction of
C. sinensis infected persons has been widely used for epidemiological studies, or for diagnosis at hospitals in Korea (
Ahn et al., 1975). Also, the enzyme-linked immunosorbent assay (ELISA) has been reported useful in terms of its high sensitivity and feasibility (
Lee et al, 1981). It is likely that ELISA is the most popular immunological technique used for diagnosis of clonorchiasis at present.
In addition, the immunoblot analysis has been used to identify antigenic proteins of
C. sinensis. Seven major IgE-reacting
C. sinensis antigens with a molecular weight from 66 to 15.5 kDa were noticed (
Yong et at, 1990 &
1993). Seven kDa and 12.5 kDa species-specific antigen were reported as the marker antigens of an active clonorchiasis (
Kim, 1994 &
1998). Hong et al. (
1997) reported that the protein bands of 43, 34 and 28-25 kDa of
C. sinensis were species-specific, and strongly reacted with the infected human sera. These studies showed that there were diverse antigenic proteins in
C. sinensis worm extract.
The purpose of the study was the production of a recombinant antigen by applying molecular biology techniques for improving a specificity or sensitivity of the conventional immunodiagnostic test using a worm extract for the antigen. Here we describe the isolation of C. sinensis cDNA encoding an antigenic protein which reacts with IgG antibodies in an experimentally infected rabbit serum, and the application of an immunodiagnostic test using a recombinant protein for diagnosis of clonorchiasis.
MATERIALS AND METHODS
Collection of parasites and sera
Clonorchis sinensis metacercariae were collected from naturally infected fish (
Pseudorasbora parva) caught in the Nakdonggang (River), Korea. These metacercariae were orally administered to experimental rabbits. Eight weeks later, adult
C. sinensis worms were obtained from the bile ducts of the rabbits, and blood collection was done. Sera were separated, and stored at -70℃ until used. Human sera of tissue-invading helminth infections were employed from the sera stock of the Department of Parasitology, Yonsei University College of Medicine (
Yong et al., 1991).
Construction of cDNA expression library
Clonorchis sinensis mRNA was purified by using the Messenger RNA isolation kit (Stratagene, La Jolla, CA, USA). In brief, live C. sinensis worms recovered from an infected rabbit were lysed completely in a denaturing solution of 4 M guanidium isothiocyanate and 0.14 M β-mercaptoethanol, and centrifuged at 12,000 g for 10 min. The supernatant was transferred to the tube containing oligo (dT) cellulose resin. The resin was washed with high-salt buffer, and poly (A)+ mRNA was eluted with low-salt buffer.
A cDNA library of C. sinensis was constructed using a ZAP Express™ cDNA Gigapack II gold cloning kit (Stratagene) according to the manufacturer's instruction. Briefly, the first strand cDNA was synthesized on 5 µg of C. sinensis poly (A)+ mRNA, and second strand was synthesized by nick translation. EcoR I adaptors were ligated to the blunt ends. Xho I restriction enzyme digestion resulted directional cDNA. The cDNA was inserted into predigested ZAP express vector arms. Packaging was carried out in vitro with Gigapack II packaging extract. The library was plaqued on Escherichia coli XLl-Blue MRF', and the number of recombinant was counted.
Immunoscreening and in vivo excision
Expression of fusion protein was induced with 2.5 mM IPTG, and transferred to the nitrocellulose membrane. Immunoscreening was carried out by phage dot immunoblot analysis using a picoBlue™ antibody screening kit (Stratagene) with a 1:500 dilution of an C. sinensis infected rabbit serum and alkaline phosphatase labelled anti-rabbit IgG. Positive clones were rescreened until all plaques on the plate were positive. One strongly reacted clone was selected. The selected clone in ZAP express vector was excised in vivo with ExAssist helper phage (Stratagene).
Immunoblot analysis
Crude extract of recombinant bacteria induced with 2.5 mM IPTG was electrophoresed in 12% SDS-polyacrylamide gels, and electrotransferred to the nitrocellulose membrane (
Tsang et al., 1983). The membranes were treated as described above for immunoscreening.
Plasmid was purified from transformed bacteria by the alkaline-SDS minipreparation method (
Sambrook el al., 1989). The cDNA insert size was determined by digestion with
EcoR I and
Xho I endonucleases, and 0.8% agarose gel electrophoresis. DNA sequencing was performed by the dideoxy nucleotide chain termination method (
Sanger et al., 1977) using a T7 Sequenase version 2.0 DNA sequencing kit (Amersham Life Science, Cleveland, OH, USA).
Total RNA of C. sinensis or Paragonimus westermani adult worms were purified using an RNA isolation kit (Stratagene) with some modifications. Each RNA was blotted on Nitran-Plus membrane (Schleicher & Schuell, Keene, NH, USA) using a slot blot apparatus (Hoefer Pharmacia Biotech. San Francisco, CA, USA).
Membranes were prehybridized in hybridization buffer (50% formamide, 5× SSPE, 2× Denhard's reagent, and 0.1% SDS) for 2 hrs at 42℃. For making the DNA probe, a cDNA insert of pBCs31 was purified from low melting-agarose gel following digestion with
EcoR I and
Xho I restriction enzymes. The purified cDNA was labeled with [α-
32P] dATP using a Random primer DNA labelling kit (Takara Shuzo, Otsu, Japan), and added to hybridization buffer. After 18 hrs of hybridization at 42℃, the nitrocellulose membrane was washed, and exposed to X-ray film (
Bok et al., 1995).
Bacteria were cultured and induced with IPTG. The recombinant protein fused to β-galactosidase was purified by an affinity chromatography using anti-β-galactosidase antibody (ProtoSorb lacZ immunoaffinity adsorbent; Promega, Madison, WI, USA) according to the manufacturer's instructions. ELISA was carried out with purified recombinant antigen (2 µg/ml) coated on ELISA plates as described (
Yong et al., 1993). The plates were reacted against a 1:200 dilution of human sera with various parasitic infections including 20 clonorchiasis, 10 paragonimiasis, 10 cysticercosis or 6 sparganosis, and developed with ortho-phenylenediamine (Sigma, St. Louis, MO, USA).
RESULTS
Immunoscreening of cDNA library and immunoblot
About 2 × 10
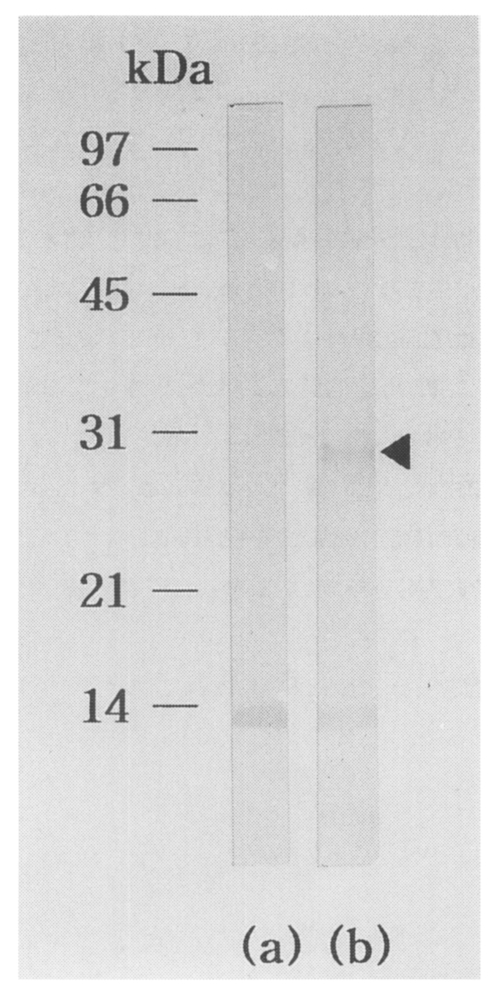
5 independent phages were screened. More than a hundred plaques showed strong positive reactions by first immunoscreening. Of these, one predominantly reacted plaque, pBCs31, was selected. IgG-immunoblot using an infected rabbit serum against recombinant bacterial extract of the clone (pBCs31) identified a reacting band of a fusion protein with a molecular mass of 28 kDa (
Fig. 1). Background reacting bands with about 14 kDa were noticed in both lane (a) and (b) of the rabbit serum against a
E. coli protein.
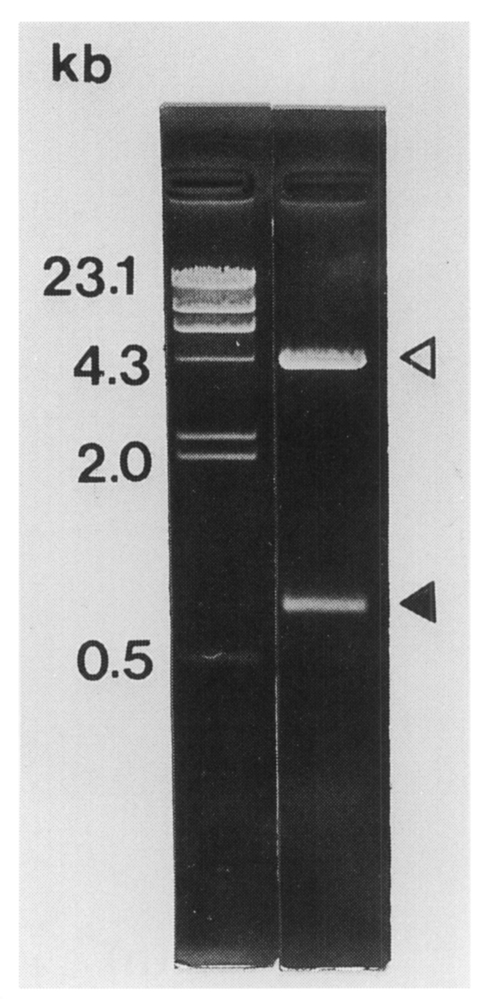
The
C. sinensis cDNA insert size contained in pBCs31 was 0.8 kb on agarose gel electrophoresis (
Fig. 2). The insert encoded a polypeptide of 192 amino acids with a predicted molecular mass of 21.1 kDa. The nucleotide sequence of the cDNA insert of pBCs31 showed an open reading frame encoding a protein bearing repetitive amino acid sequence (
Fig. 3). The nucleotide sequence has been submitted to the GenBank under an accession number AF077549 through BRIC (biological information center), Korea. The cloned gene was composed of 16 copies of a 30 base pair repeat and an additional 320 bases at its 3'-end. a partial cDNA fragment without start codon. The deduced amino acid sequence of the tandem repeat was AQPPKSGDGG. A homology search in GenBank using NCBI Blast program showed that this putative protein has 58% identity with
Xenopus laeuis APEG protein at amino acid level (ACC#: X51394).
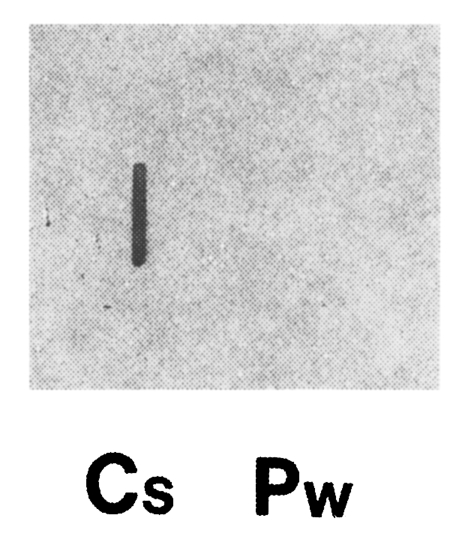
Clonorchis sinensis adult worm RNA showed a positive reaction with the cloned gene, while
P. westermani adult worm RNA showed no reactions by RNA slot blot analysis (
Fig. 4). This result along with the immunoblot analysis (
Fig. 1), confirmed that the cloned gene in the study was reverse transcribed from
C. sinensis adult mRNA.
Purification of recombinant antigen and ELISA
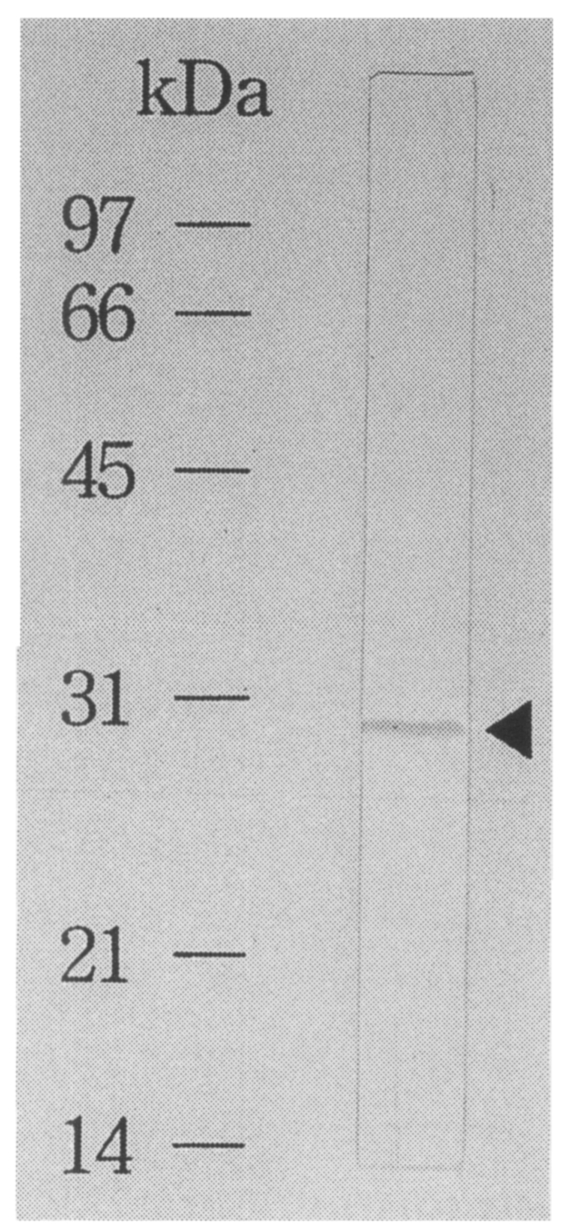
The recombinant antigen was purified by using affinity chromatography as pure as a single band on immunoblot using a
C. sinensis infected rabbit serum (
Fig. 5). By ELISA using the fusion protein as an antigen, 55% of 20 sera of
C. sinensis egg passers showed positive reactions, while the conventional ELISA using a crude antigen of
C. sinensis adult worm showed positive reactions in 70%. So, sensitivity of the ELISA decreased. No false positive cases were found of any sera with 10 paragonimiasis, 6 cysticercosis or 10 sparganosis in the study using the fusion protein as an antigen. By the conventional ELISA, 10% of 20 paragonimiasis cases showed false positive reactions. In summary, ELISA using a purified species-specific recombinant protein showed high specificity for diagnosis of clonorchiasis (
Table 1).
DISCUSSION
We have been able to select a clone producing recombinant
C. sinensis protein from a
C. sinensis cDNA expression library. The cloned antigen coding gene is a little unique in terms of being composed of tandemly repeated nucleotide sequences. However, it seems likely that many of the antigen coding genes or surface antigen genes in a parasite genome may be organized in tandem repetitions (
Dame et al., 1984;
Peterson et al., 1986). Tandemly repeated peptide antigens may induce a strong humoral immune response in the infected hosts.
Xenopus APEG protein homologous to pBCs31 was composed of repetitive amino acids including alanine, proline, glutamic acid and glycine. It is a component of storage granules contained in the dermal secretion gland of a frog (
Gmachl et al., 1990). Although some homology of the gene was found, more study is needed of the molecule to elucidate its function and any relationship between the two.
Cross-reactions have been frequently reported and known well in the immunodiagnosis of clonorchiasis and paragonimiasis (
Cho and Soh, 1976;
Soh et al., 1985;
Yong et al., 1991). In order to improve specificity of the ELISA for serodiagnosis of clonorchiasis, monoclonal antibodies were produced, and applied in previous studies. About one fourth of monoclonal antibodies were species-specific, but others showed some degree of cross reactivity against various helminths' crude antigens (
Yong et al., 1990 &
1991). A species-specific monoclonal antibody-based competitive ELISA test for diagnosis of clonorchiasis showed better specificity than the conventional ELISA. However, it would not be practically useful because of the limited availability of highly specific monoclonal antibodies and the cumbersome standardization of the test. The production of recombinant
C. sinensis antigens in
E. coli in this study suggests another way to increase specificity of the immunological test. If techniques are readily available for mass production and feasible purification, it can be quite useful theoretically and practically. Actually the amount of recombinant protein production achieved in the study was not much at this moment, although the recombinant protein was found to be soluble. Subcloning to another cloning vector,
i.e. pET-23b (Novagen, Madison, WI, USA), was tried to solve the problem without any improvement of the production (data not shown).
The intradermal test for diagnosis of
C. sinensis infection or
P. westermani has been widely used since the 1960s (
Kim and Yang, 1964) in spite of its well-known limitations. False positive reactions or prolonged response even after cure are quite common instances when we perform the intradermal test. Cross-reactive IgE-reacting molecules between
C. sinensis and
P. westermani were identified by IgE-immunoblot and autoradiography (
Yong et at, 1993). Conventionally, in order to produce antigens for the intradermal test, we need to catch infected fish in the river, infect experimental rabbits, collect the worms from sacrificed animals, and extract the crude antigen from adult worms. Mass production of highly species-specific recombinant proteins would be a convenient way of obtaining antigens for immunodiagnosis. Production of more sensitive and species-specific recombinant antigen for intradermal test would be a promising subject for study in the near future.
So far, there have been very few papers on the molecular characteristics of
C. sinensis antigens (
Hong, 1993). Although a cDNA clone of
C. sinensis was selected for immunodiagnosis of clonorchiasis in this communication primarily, we believe that this kind of DNA approach is indispensable for dissecting and identifying
C. sinensis antigens in the future.
Notes
-
This study was supported by Non-Directed Research Fund, Korea Research Foundation, 1996.
ACKNOWLEDGEMENTS
We sincerely thank to Professor Dong-Il Chung and his colleagues. Department of Parasitology, Kyungpook National University School of Medicine, Taegu, Korea, for their assistance in catching freshwater fish harboring C. sinensis metacercariae.
References
- 1. Ahn YK, Soh CT, Han JK. Comparison of dermal reaction with VBS and KST antigens of Clonorchis sinensis in reference to sensitivity and specificity. New Med J 1975;18(9):1195-1201.
- 2. Bok JW, Jin YJ, Lee JH. Independent mechanisms are utilized for the coordinate and transient accumulation of two differentiation-specific mRNAs during differentiation of Naegleria gruberi amoebae into flagellates. Exp Cell Res 1995;219:47-53.
- 3. Cho KM, Soh CT. Indirect fluorescent antibody test for the serodiagnosis of paragonimiasis and clonorchiasis. Yonsei Rep Trop Med 1976;7(1):26-39.
- 4. Dame JB, Williams JL, McCutchan TF, et al. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science 1984;225(4662):595-599.
- 5. Gmachl M, Berger H, Thalhammer J, Kreil G. Dermal glands of Xenopus laevis contain a polypeptide with a highly repetitive amino acid sequence. FEBS Letters 1990;260(1):145-148.
- 6. Hong SJ. Clonorchis sinensis tropomyosin: Cloning and sequence of partial cDNA amplified by PCR. Korean J Parasitol 1993;31(3):285-292.
- 7. Hong ST, Kho WG, Lee MJ, Lee JS, Lee SH. Immunoblot patterns of clonorchiasis. Korean J Parasitol 1997;35(2):87-93.
- 8. Kim DC, Yang HK. Epidemiological study of paragonimiasis and clonorchiasis on Cheju-Do. Report NIH, Korea 1964;1(1):98-104.
- 9. Kim SI. Immune reactions between excretory-secretory antigens and specific antibodies of Clonorcflis sinensis before and after praziquantel treatment in experi-mentally infected rabbits. Korean J Parasitol 1994;32(1):35-42.
- 10. Kim SI. A Clonorchis sinensis-specific antigen that detects active human clonorchiasis. Korean J Parasitol 1998;36(1):37-45.
- 11. Lee JK, Min DY, Im KI, Lee KT, Soh CT. Study of enzyme-linked immunosorbent assay (ELISA) method in serodiagnosis of Clonorchis sinensis infection. Yonsei J Med Sci 1981;14(1):133-147.
- 12. Peterson DS, Wrightsman RA, Manning JE. Cloning of a major surface antigen gene of Trypanosoma cruzi and identification of a nonapeptide repeat. Nature 1986;322:566-568.
- 13. Rim HJ. The current pathobiology and chemotherapy of clonorchiasis. Korean J Parasitol 1986;24(suppl):1-141.
- 14. Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A laboratory manual. 1989, 2nd ed.. NY, USA. Cold Spring Harbor.
- 15. Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 1977;74:5463-5467.
- 16. Soh CT, Min DY, Ryu JS, Yong TS. Study on the reproducibility of ELISA technique for the diagnosis of clonorchiasis and paragonimiasis. Yonsei Rep Trop Med 1985;16(1):1-10.
- 17. Tsang VCW, Peralta JM, Simons AR. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol 1983;92:377-391.
- 18. Yong TS, Im KI, Chung PR. Analysis of Clonorchis sinensis antigens and diagnosis of clonorchiasis using monoclonal antibodies. Korean J Parasitol 1991;29(3):293-310.
- 19. Yong TS, Im KI, Lee OY, Kim DC. Evaluation of Clonorchis sinensis specific antigen purified by affinity chromatography using monoclonal antibodies for serodiagnosis of human clonorchiasis. Yonsei Rep TropMed 1990;21:25-32.
- 20. Yong TS, Kim DS, Lee SY, Im KI, Lee KY. Detection of specific serum IgE in clonorchiasis cases and analysis of Clonorchis sinensis allergens. Yonsei Med J 1993;34(3):248-257.
- 21. Yong TS, Shin CO, Im KI. Demonstration of immune responses in rabbits infected with Clonorchis sinensis by enzyme-immunoelectrotransfer blot. Yonsei Rep Trop Med 1990;21:19-24.
Fig. 1IgG-immunoblot analysis of pBCs31 fusion protein. The pBCs31 induced in E. coli XLl-Blue MRF' shows 28 kDa band reactive to an C. sinensis infected rabbit serum, (a) Negative control of E. coli lysate containing pBK-CMV vector only, (b) E. coli lysate containing induced pBCs31. At about 14 kDa, background reacting bands against a E. coli protein were noticed in both (a) and (b).

Fig. 2The size of C. sinensis cDNA insert contained in pBCs31 was 0.8 kb (closed arrowhead) on 0.8% agarose electrophoresis following double digestable with EcoR I and Xho I. Open arrowhead shows the vector.
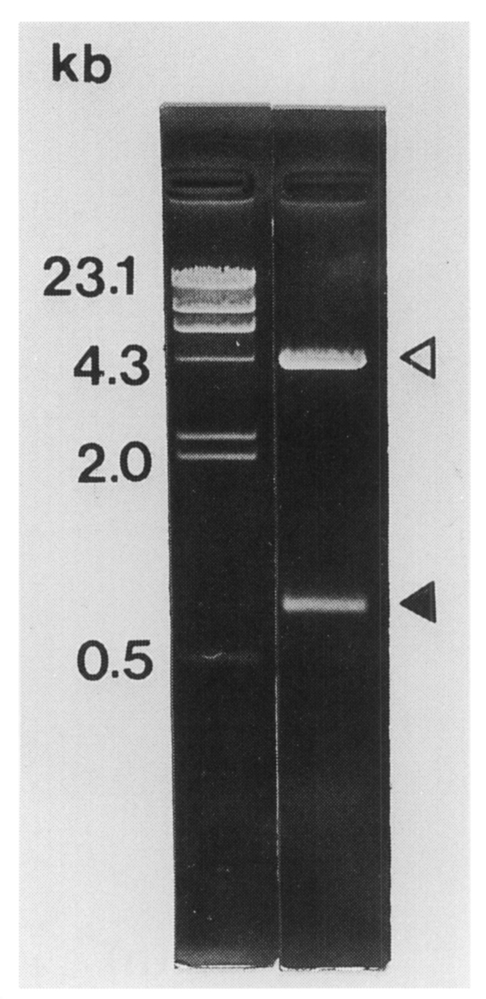
Fig. 3The nucleotide sequence of pBCs31 insert and deduced amino acid sequence showed that one open reading frame encoding an antigenic protein bearing repetitive epitopes. The indicated reading frame is in phase with the β-galactosidase gene of pBK-CMV vector, and G in the parenthesis at the beginning is from the adaptor sequence for cloning.

Fig. 4Total RNAs of adult C. sinensis and P. westermani (5 µg each) were blotted, and hybridized with 32P-labelled cloned gene (i.e., C. sinensis cDNA contained in pBCs31). Clonorchis sinensis RNA showed a positive reaction, while P. westermani RNA showed no reaction.
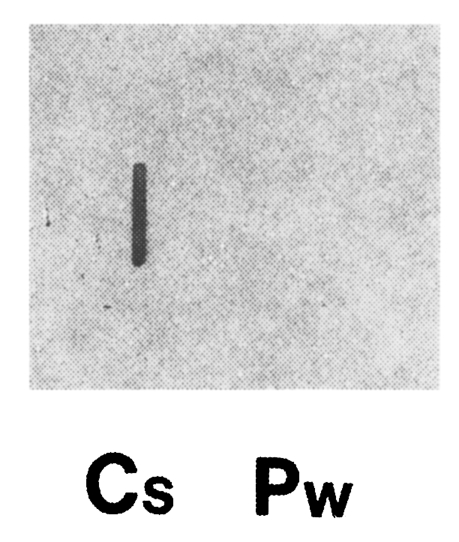
Fig. 5Immunoblot analysis of an affinity purified recombinant protein. The recombinant fused protein to β-galactosidase shows a band reacting to the C. sinensis infected sera.
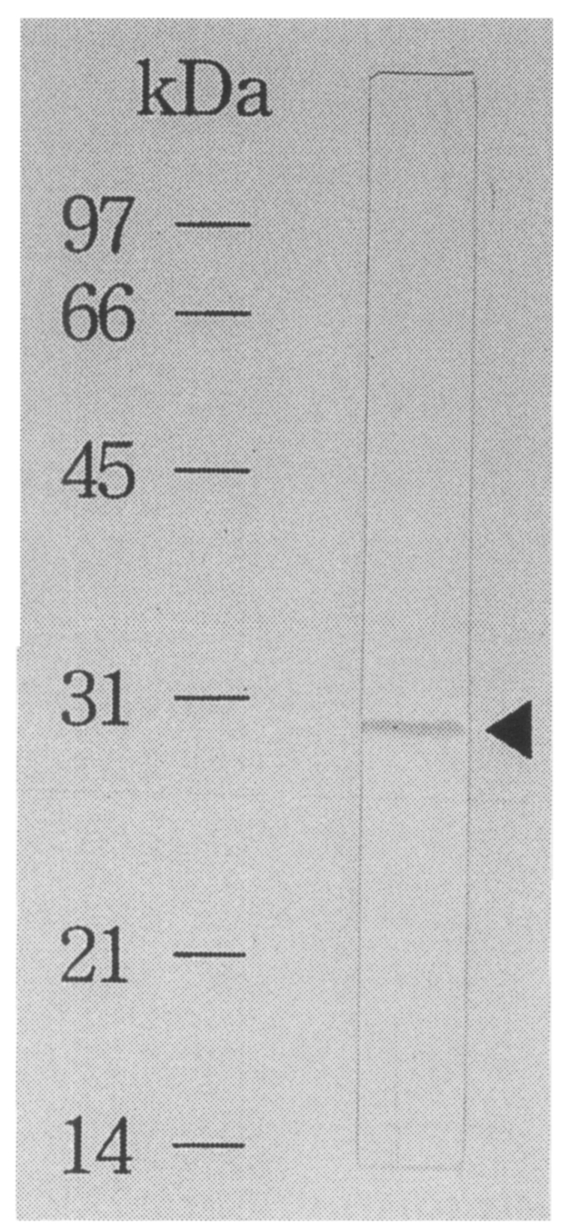
Table 1.Reactivity of pBCs31 recombinant protein to sera from cases with various parasitic infections or negative control by ELISA
Table 1.
|
Source of human sera |
No. of cases assayed |
No. of cases to pBCs31 |
No. of cases anti-C. sinensis adult worm antigen positive |
|
clonorchiasis |
20 |
11 |
14 |
|
paragonimiasis |
10 |
0 |
2 |
|
cysticercosis |
10 |
0 |
0 |
|
sparganosis |
6 |
0 |
0 |