Abstract
Several studies have shown the mechanisms and importance of immune responses against Toxoplasma gondii infection and the notable role of cholinesterases in inflammatory reactions. However, the association between those factors has not yet been investigated. Therefore, the aim of this study was to evaluate the acetylcholinesterase (AChE) activity in blood and lymphocytes and the activity of butyrylcholinesterase (BChE) in serum of rats experimentally infected with T. gondii during the acute phase of infection. For that, an in vivo study was performed with evaluations of AChE and BChE activities on days 5 and 10 post-infection (PI). The activity of AChE in blood was increased on day 5 PI, while in lymphocytes its activity was enhanced on days 5 and 10 PI (P<0.05). No significant difference was observed between groups regarding to the activity of BChE in serum. A positive (P<0.01) correlation was observed between AChE activity and number of lymphocytes. The role of AChE as an inflammatory marker is well known in different pathologies; thus, our results lead to the hypothesis that AChE has an important role in modulation of early immune responses against T. gondii infection.
-
Key words: Toxoplasma gondii, acute toxoplasmosis, acetylcholinesterase (AchE), butyrylcholinesterase (BchE)
INTRODUCTION
Toxoplasma gondii is a ubiquitous coccidian parasite of the Phylum Apicomplexa, the largest and most important group of obligate parasites. It is unusual within this group in its capacity to parasitize a diverse array of cell types and infect virtually any warm-blooded animal [
1].
T. gondii is a remarkably successful organism; around one-third of the world's population is seropositive for this parasite. Seroprevalence increases with age and varies around the world [
2]. Thus, in the USA, the overall seroprevalence is around 20-25% but in El Salvador, France, and Brazil, for example, it may be as high as 75-80%.
Exposure to pathogenic threats activates inflammatory reactions, followed by a fast and crucial anti-inflammatory responses [
3]. This limits the inflammatory processes below a certain threshold, ascertaining survival and avoiding autoimmune diseases or spreading of the inflammatory components into the bloodstream, which may lead to septic shock [
3]. Primary infections with
T. gondii stimulate production of high levels of interleukins, such as IL-12 and IFN-γ, by cells of the innate immune system, being central efforts to resistance to the disease [
4]. In recent years, descriptions about the participation of the cholinergic system in the inflammatory response has been described [
5-
7].
Cholinergic signaling is notably involved in anti-inflammatory reactions [
8] and cholinesterase enzymes are present in cholinergic and non-cholinergic tissues. They are divided into 2 classes, i.e., acetylcholinesterase (AchE) and butyrylcholinesterase (BChE) according to their catalytic properties and specificity for substrates, sensitivity to inhibitors, and tissue distribution [
9,
10]. AChE and BChE both catalyse the hydrolysis of the neurotransmitter acetylcholine (ACh), a fundamental process in regulating the cholinergic system [
10,
11]. AChE (EC 3.1.1.7) is a membrane-bound enzyme found mainly in the brain, muscles, erythrocytes, lymphocytes and cholinergic neurons [
9,
12] that preferentially hydrolyses esters with acetyl group. Thus, the vagus nerve releases acetylcholine (ACh) when stimulated (either electrically or pharmacologically), inhibiting activation of macrophages and release of pro-inflammatory cytokines, e.g., IL-6, tumor necrosis factor alpha (TNF-α), IL-1, and IL-18. The lymphocytes synthesize ACh, which is degraded by AChE, and in consequence of this, the complete cholinergic repertoire of immune cells was termed the lymphocytic cholinergic system [
9].
Based on the knowledge that toxoplasmosis has a high stimulation on the immune system [
4], added to the immune modulator role of the cholinergic system, the aim of this study was to evaluate the AChE activities in blood and lymphocytes, as well as BChE activities in serum of rats experimentally infected with
T. gondii during the acute phase of infection. Rats were chosen because they are more resistant to toxoplasmosis, providing conditions to investigate the possible participation of cholinesterases in the immune response during the acute phase of the disease.
MATERIALS AND METHODS
Ethics statement
The procedure was approved by the Animal Welfare Committee of Centro Universitário Fransciscano (UNIFRA), number 003/2011, Brazil. Experiments were performed according to the Brazilian regulations for experimentation animals (CONCEA). All efforts were made to minimize suffering of animals.
Experimental animals
Twenty-four adult male Wistar rats (Rattus norvegicus), averaging 60 days old and 200 g in weight, from the central vivarium of the Universidade Federal de Santa Maria (UFSM), Brazil, were used. The animals were kept in an experimental room with controlled temperature and humidity (25℃; 70% relative humidity). They were fed a commercial ration, with water ad libitum, and submitted to a period of 7 days of adaptation.
T. gondii strain and preparation of inoculum
For this experiment, T. gondii (RH strain, isolated by Sabin, 1941) kept in liquid nitrogen in the laboratory was utilized. Firstly, one mouse was inoculated with tachyzoites of T. gondii. Four days later, the peritoneal fluid containing the parasite was collected and inoculated into other mice. This procedure was repeated 3 times, in order to reactivate the parasite virulence and for obtaining a large number of tachyzoites of T. gondii.
Experimental design and sample collection
The animals were divided in 2 groups (A and B), 12 animals per each group, and from these again they were subdivided into 4 subgroups (A1, A2, B1, and B2), 6 animals per each group). Groups A and B served for uninfected and infected controls, respectively. A volume of 0.5 ml of peritoneal fluid containing 1.2×107 tachyzoites was inoculated to group B (12 rats) intraperitoneally. The volume of 0.5 ml saline was administered to group A by the same route.
On day 5 post infection (PI) (subgroup A1 and B1) and day 10 PI (subgroup A2 and B2), sample collections were performed. The animals were anesthetized in chamber with isoflurane for collection of blood by cardiac puncture (8 ml). The storage of the samples was considered accordingly to the analysis. Thus, a part of the material collected was allocated in tubes containing anticoagulant (EDTA 10%) for separation of lymphocytes (4 ml) and analysis of hemogram and blood AChE (1 ml). The volume of 3 ml was stored in a tube without anticoagulant to obtain serum for cytokine and BChE analysis.
Hematological evaluation and cytokines
Complete blood count and hemoglobin determination were performed using an automated cell counter (Vet Auto Haematology Analyser, model BC 2800, Nanshan, Shenzhen, China). For morphological evaluation of the blood and differential count of white blood cells, blood smears were stained using a Diff-Quik commercial kit and visualized under the microscope. Mean corpuscular volume and mean corpuscular hemoglobin concentrations were calculated according to Feldman et al. [
13].
Quantification of cytokines was performed in order to establish occurrence of immune responses against infection with T. gondii, supporting our findings on the cholinesterase. The quantification of pro-inflammatory cytokines was performed by ELISA using commercial kits for rat IFN-γ, TNF-α, IL-1, and IL-6 (eBIOSCIENCE, San Diego, California, USA), according to manufacturer's instructions.
Lymphocyte separation
Lymphocytes were also obtained from whole blood with EDTA by gradient separation using Ficoll-Histopaque™ plus, according to the technique described by Böyum [
14]. After separation, lymphocyte viability and integrity were confirmed by determining the percentage of cells excluding 0.1% trypan blue and measuring the lactate dehydrogenase activity [
15]. Protein concentrations of the lymphocytes were determined by the Coomassie blue method [
16] using bovine serum albumin as the standard.
Blood samples were diluted 1:50 (v/v) in lysis solution (0.1 mmol/L potassium/sodium phosphate buffer containing 0.03% Triton X-100) to determine AChE activities. Subsequently, the samples were frozen for 10 days and then processed. The AChE enzymatic assay in total blood was done by the method of Ellman et al. [
17] as modified by Worek et al. [
18]. The incubation system was composed of 0.1 mol/L sodium phosphate buffer (pH 7.4), 5,59-dithiobis-(2-nitrobenzoic acid) (DTNB) 10 mmol/L for hemolysis of blood. The increase in absorbance was registered over 2 min at 436 nm. The specific activities of whole-blood AChE were calculated from the quotient between AChE activity and hemoglobin (Hb) concentration and the results were expressed as µm/Umol of Hb.
After isolation of the lymphocytes, AChE activities was determined according to the method described by Fitzgerald and Costa [
19]. Briefly, proteins of all samples were adjusted to 0.1-0.2 mg/ml, and 0.2 ml of intact cells was added to a solution containing 1.0 mmol acetylthiocholine (AcSCh), 0.1 mmol DTNB, and 0.1 mmol phosphate buffer (pH 8.0). Immediately before and after incubation for 30 min at 27℃ the absorbance was read on a spectrophotometer at 412 nm. The AChE activity was calculated from the quotient between the lymphocyte AChE activity and protein content. The results were expressed as µmol AcSCh mg of protein.
The BChE enzymatic assay in the serum was determined by method of Ellman et al. [
17] using the substrate butyrylthiocholine. The sample was pre-incubated at 37℃ for 2 min, and reading was performed for 2 min at intervals of 20 sec on a spectrophotometer at 412 nm. The sample analysis was carried out in duplicate, and the enzyme activity was expressed in µmoles BcSCh/h/mg of protein.
The data were submitted to an analysis of variance followed by the Student's t-test (P<0.05). The effect of AChE activity in lymphocytes on the number of lymphocytes was analyzed by linear correlation. The analyses were performed using SAS statistical package (SAS Institute, Cary, North Carolina, USA) with a significance level of 5% (P<0.05).
RESULTS
Course of infection, hematologic parameters, and cytokines
During the infection, clinical manifestations and changing the behavior of animals were not observed in both groups. On day 5 PI, there were no significant (
P>0.05) alterations in hematological parameters between groups. The number of erythrocytes and hemoglobin concentration were decreased (
P<0.05) in the infected groups on day 10 PI, whereas total leukocytes and lymphocytes presented an increase in their levels when compared to the control group (
Table 1).
In this study, we found that T. gondii infection in rats led to the occurrence of an inflammatory process. This is because the IL-1, IL-6, INF-γ, and TNF-α levels were increased on days 5 and 10 PI in all groups when compared to the control group (P<0.05).
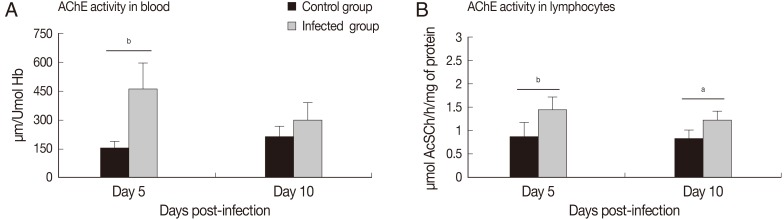
AChE activities in total blood and lymphocytes
On day 5 PI, the activity of AChE in total blood increased significantly (
P<0.01) in the infected group, compared to the control group. However, on day 10 PI, no differences were observed between infected and control groups (
Fig. 1A). AChE activities in lymphocytes were increased in both moments, days 5 (
P<0.01) and 10 (
P<0.05) PI, in the infected groups, in comparison with the control group (
Fig. 1B).
Statistical analysis demonstrated a positive correlation (r=0.67) between the number of lymphocytes and AChE activity in lymphocytes on day 10 PI (P<0.01). However, on day 5 PI, no correlation was observed.
BChE activities in serum
No significant (P>0.05) difference was observed in the BChE activities in serum of rats infected with T. gondii compared to healthy subjects in both periods evaluated.
DISCUSSION
T. gondii can invade every nucleated cell in the body, although the preferred target organs are lymph nodes, brain, heart, and lungs. Proliferation of tachyzoites results in infection of neighboring cells and necrosis, which can be associated with an intense mononuclear cell reaction [
20]. Our data demonstrated that the number of lymphocytes, as well as total leukocytes, were increased during the second sampling period, probably in response to the tachyzoite proliferation during the infection. In this study, we have demostrated the relationship of the cholinergic system with white blood cell proliferation during the acute phase of an experimental infection with
T. gondii in rats.
It is well established and several investigations proved that blood cells possess a cholinergic system consisting of ACh muscarinic and nicotinic receptors (mAChR and nAChR, respectively), choline acetyltransferase (ChAT), and acetylcholinesterase [
9,
21]. Our results show an increase in the whole blood (day 5 PI) and lymphocyte (days 5 and 10 PI) AChE activities. The activity of AChE in the whole blood was increased on day 5 PI during the period of major parasitemia. Our hypothesis is that the elevation of its activity is directly related to an increased expression of the enzyme in erythrocytes as a response to a high parasitemia of the period. Our results are in accordance with Szelenyi [
22], who described a significant increase in AChE activity and mAChR expression in normal human peripheral blood lymphocytes as an early response to various stimuli. While the major expression and/or activity of the enzyme was supposedly on day 5 PI protecting the cells, the anti-inflammatory action simultaneously occurred, partially cleaning the parasite from the bloodstream, however, also reducing the erythrocytes and hemoglobin levels.
In both phases (days 5 and 10 PI), an increase was observed in the AChE activity in lymphocytes when compared to not-infected animals. When there is an increase in the AChE activity, a rapid degradation of ACh occurs which is considered a molecule with anti-inflammatory action, since it binds to nicotinic receptors in lymphocyte surfaces, and thus inhibits the production of cytokines, serotonin, histamine, nitric oxide, lysosomal enzymes, prostaglandins, and leukotrienes which are among the mediators of the inflammatory process (7,9,23,24). It is noteworthy that the animals in this study presented increased levels of pro-inflammatory cytokines We found that the increase in AChE activity in lymphocytes was associated to lymphocytosis (day 10 PI), a pro-inflammatory action in order to reduce the free ACh. It is known that ACh is produced within the lymphocytes [
9]. Therefore, increasing the number of lymphocytes certainly increases the concentration of the free ACh, and consequently increases the AChE activity as an inflammatory action.
In this study, using rats as an experimental model, we did not found variation in the activity of BChE between infected and uninfected groups. Therefore, the enzyme BChE seems not to be involved in immune responses during acute toxoplasmosis in rats, unlike what happened with AChE. However, when the activity of BChE was evaluated in serum and liver of mice infected with
T. gondii (an experimental model highly susceptible to the parasite), a reduction in this enzymatic activity was observed, associated with liver lesions, the major organ responsible for BChE synthesis [
25]. Therefore, we believe that the difference between the studies is co-related to the experimental model used, leading to the conclusion that alteration in the levels of this enzyme is much more related to the liver injury than immune responses.
The results obtained in the present study demonstrated alterations in the activity of AChE in whole blood and lymphocytes associated with the increase of total leukocytes by lymphocytosis. AChE had its activity enhanced in lymphocytes, as well as total blood, may providing a pro-inflammatory action in order to reduce the free ACh, a molecule which has an anti-inflammatory action. Therefore, our results lead to the hypothesis that AChE has an important role in early modulation of immune responses against T. gondii during acute infection through an inflammatory effect, contributing to the response against the parasite. On the other hand, BChE seems not to be involved in the immune response of rats with acute toxoplasmosis.
References
- 1. Dubey JP. The history and life cycle of Toxoplasma gondii. In Weiss LM, Kim K eds, Toxoplasma gondii, the Model Apicomplexan: Perspectives and Methods. London, UK. Academic Press; 2007, pp 1-17.
- 2. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004;363:1965-1976.
- 3. Tracey KJ. The inflammatory reflex. Nature 2002;420:853-859.
- 4. Miller CM, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol 2009;39:23-39.
- 5. Da Silva AS, Monteiro SG, Gonçalves JF, Spanevello R, Schmatz R, Oliveira CB, Costa MM, França RT, Jaques JA, Schetinger MR, Mazzanti CM, Lopes ST. Trypanosoma evansi: Immune response and acetylcholinesterase activity in lymphocytes from infected rats. Exp Parasitol 2011;127:475-480.
- 6. Da Silva CB, Wolkmer P, Da Silva AS, Paim FC, Tonin AA, Castro VS, Felin DV, Schmatz R, Gonçalves JF, Badke MR, Morsch VM, Mazzanti CM, Lopes ST. Cholinesterases as markers of the inflammatory process in rats infected with Leptospira interrogans serovar. icterohaemorrhagiae. J Med Microbiol 2012;61:278-284.
- 7. Das UN. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med Sci Monit 2007;13:RA214-RA221.
- 8. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458-462.
- 9. Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci 2003;74:675-696.
- 10. Kimura R, Ushiyama N, Fujii T, Kawashima K. Nicotine-induced Ca2+ signaling and down-regulation of nicotinic acetylcholine receptor subunit expression in the CEM human leukemic T-cell line. Life Sci 2003;72:2155-2158.
- 11. Li B, Stribley JA, Ticu A, Xie W, Schopfer LM, Hammond P, Brimijoin S, Hinrichs SH, Lockridge O. Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. J Neurochem 2000;75:1320-1331.
- 12. Schetinger MRC, Porto NM, Moretto MB, Morsch VM, Da Rocha JBT, Vieira V, Moro F, Neis RT, Bittencourt S, Bonacorso HG, Zanatta N. New benzodiazepines alter acetylcholinesterase and ATPDase activities. Neurochem Res 2000;25:949-955.
- 13. Feldman BF, Zinkl JG, Jain NC. Schalm's Veterinary Hematology. 5th ed. Philadelphia, USA. Lippincott Williams & Wilkins; 2000.
- 14. Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 1968;97:77-89.
- 15. Strober W. Trypan blue exclusion test of cell viability. In Coligan J, Kruisbeek A, Marguiles D, Shevach E, Strober W eds, Current Protocols in Immunology. 2001, pp A.3B.1-A.3B.2.
- 16. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-254.
- 17. Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88-95.
- 18. Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta 1999;288:73-90.
- 19. Fitzgerald BB, Costa LG. Modulation of muscarinic receptors and acetylcholinesterase activity in lymphocytes and in brain areas following repeated organophosphate exposure in rats. Fundam Appl Toxicol 1993;20:210-216.
- 20. Evans TG, Schwartzman JD. Pulmonary toxoplasmosis. Semin Respir Infect 1991;6:51-57.
- 21. Tayebati SK, El-Assouad D, Ricci A, Amenta F. Immunochemical and immunocytochemical characterization of cholinergic markers in human peripheral blood lymphocytes. J Neuroimmunol 2002;132:147-155.
- 22. Szelényi J, Páldi-Haris P, Hollán S. Changes in the cholinergic system of lymphocytes due to mitogenic stimulation. Immunol Lett 1987;16:49-54.
- 23. Czura CJ, Tracey KJ. Autonomic neural regulation of immunity. J Intern Med 2005;257:156-166.
- 24. Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I, Brenner T. Anti-inflammatory properties of cholinergic up-regulation: a new role for acetylcholinesterase inhibitors. Neuropharmacology 2006;50:540-547.
- 25. Da Silva AS, Tonin AA, Thorstenberg ML, Leal DB, Fighera R, Flores MM, França RT, Camillo G, Vogel FS, de la Rue M, Lopes ST. Relationship between butyrylcholinesterase activity and liver injury in mice acute infected with Toxoplasma gondii. Pathol Res Pract 2013;209:95-98.
Fig. 1AChE activity in blood (A) and lymphocytes (B) of rats infected with Toxoplasma gondii (days 5 and 10 PI) compared not-infected (mean and SD; aP<0.05; bP<0.01).

Table 1.Hematological parameters of rats experimentally infected with Toxoplasma gondii
Table 1.
|
Parameters |
Day postinfection |
Control group (Mean ± SD) |
Infected group (Mean ± SD) |
|
Total erythrocytes (×106/μl) |
05 |
6.40 ± 0.25 |
6.10 ± 0.24 |
|
10 |
6.42a ± 0.20 |
5.80a ± 0.50 |
|
Hemoglobin (g/dl) |
05 |
12.50 ± 0.30 |
12.00 ± 0.50 |
|
10 |
12.00a ± 0.36 |
10.25a ± 0.66 |
|
MCV (fl) |
05 |
62.9 ± 1.20 |
65.0 ± 3.31 |
|
10 |
61.4 ± 2.31 |
63.0 ± 3.60 |
|
MCHC (%) |
05 |
28.4 ± 0.47 |
28.9 ± 1.3 |
|
10 |
28.2 ± 0.27 |
28.0 ± 0.40 |
|
Total leukocytes (/μl) |
05 |
3,900 ± 1,126 |
4,500 ± 1,345 |
|
10 |
4,120a ± 900 |
5,750a ± 918 |
|
Neutrophils (/μl) |
05 |
1,126 ± 650 |
945 ± 445 |
|
10 |
1,197 ± 512 |
926 ± 540 |
|
Lymphocyte (/μl) |
05 |
2,608 ± 730 |
3,480 ± 1,020 |
|
10 |
2,768a ± 995 |
4,572a ± 748 |
|
Eosinophils (/μl) |
05 |
84 ± 43 |
50 ± 32 |
|
10 |
68 ± 40 |
160 ± 104 |
|
Monocytes (/μl) |
05 |
82 ± 52 |
25 ± 15 |
|
10 |
87 ± 35 |
92 ± 61 |