Abstract
Anaplasma species are obligate intracellular pathogens that can cause tick-borne diseases in mammalian hosts. To date, very few studies of their occurrence in Korean native goats (Capra aegagrus hircus) have been reported. In the present study, we investigated Anaplasma infection of Korean native goats on Jeju Island, Republic of Korea, and performed phylogenetic analysis based on the 16S rRNA gene sequences. Our results showed that Anaplasma infection was found mostly in adult female goats. The phylogenetic tree revealed that the 7 sequences identified in Korean native goats could belong to Anaplasma sp. and were distinct from A. marginale, A. centrale, and A. ovis. The results indicated that the sequences identified to belong to Anaplasma were closely related to sequences isolated from goats in China and were clustered within the same group. To our knowledge, this is the first study to detect Anaplasma sp. infection in Korean native goats.
-
Key words: Anaplasma, Korean native goat, phylogenetic analysis, 16S rRNA gene
Anaplasmosis is a tick-borne disease caused by
Anaplasma species, which are obligate intracellular pathogens that infect humans and animals [
1,
2]. Clinical manifestations of the disease include anemia, fever, weight loss, decreased milk production, abortion, and frequently, death. The genus
Anaplasma is composed of 6 species that vary in host preference and cell tropism.
A. marginale,
A. centrale, and
A. ovis infect the red blood cells of ruminants [
3-
5].
A. bovis infects the monocytes of ruminants and small mammals [
6].
A. phagocytophilum is the causative agent of human granulocytic anaplasmosis and infects the neutrophils of humans, ruminants, dogs, and horses [
7,
8]. Finally,
A. platys is a platelet pathogen that infects dogs, and can cause canine cyclic thrombocytopenia [
9].
Of these
Anaplasma species,
A. marginale,
A. centrale, and
A. ovis are prominent pathogens in ruminants worldwide [
3,
10,
11]. In particular,
A. marginale and
A. ovis have considerable economic importance in tropical and subtropical areas [
4].
A. marginale is known to be highly pathogenic in cattle, while
A. centrale is less pathogenic.
A. ovis is moderately pathogenic in sheep, goats, and wild ruminants [
1,
12] and causes acute disease in animals exposed to stress or other predisposing factors such as hot weather, deworming, tick infestation, and animal movement [
13].
The importance of anaplasmosis in small ruminants in the Republic of Korea (ROK) is not yet known. The infection in sheep and goats is usually asymptomatic; however, it can sporadically cause hemolytic anemia and hemoglobinuria.
Anaplasma infection may likely be neglected because of its low economic importance in the goat production industry of the ROK. Although there have been previous reports of
Anaplasma infection in Korean native goats (
Capra aegagrus hircus) [
14,
15], epidemiological studies on
Anaplasma infection in these animals have not been well reported. Therefore, the objective of this study was to investigate
Anaplasma infection in Korean native goats pastured on Jeju Island where ticks are most widely distributed and to perform molecular characterization of
Anaplasma species detected on Jeju Island.
Whole blood samples from 39 Korean native goats on Jeju Island, ROK, were collected in April 2014. This herd typically grazed in the pasture for at least 1 season every year; that is, the animals were housed in stables in the cooler months (November-April), whereas in the warmer months (May-October), they grazed in the pasture. All goats were clinically healthy and no blood-sucking ticks were found on them. Blood samples were collected and immediately frozen at -80˚C until DNA extraction was performed.
Genomic DNA was extracted from whole blood samples using the DNeasy Blood & Tissue Kit (Qiagen, Valencia, California, USA) according to the manufacturer’s instructions. For the detection of Anaplasma infection, PCR was performed using the AccuPower® Rickettsiales 3-Plex PCR Kit (Bioneer, Daejeon, Korea). Specific primer sets targeting the 16S rRNA were used to detect species belonging to Anaplasma (F, 5´-TACCTCTGTGTTGTAGCTAACGC-3´; R, 5´-CTTGCGACATTGCAACCTATTGT-3´), Ehrlichia (F, 5´-CGGAATTCCTAGTGTAGAGG-3´; R, 5´-AGGAGGGATACGACCTTC AT-3´), and Rickettsia (F, 5´-TAGGGGATGATGGAATTCCTA-3´; R, 5´-CCCCCGTCA ATTCCTTTGAG-3´). The predicted sizes of the amplified PCR products for Anaplasma, Ehrlichia, and Rickettsia were 429 bp, 340 bp, and 252 bp, respectively. The following cycling conditions were used: 95˚C for 15 min; 40 cycles of 95˚C for 10 sec, 58˚C for 30 sec, and 72˚C for 30 sec; and final extension at 72˚C for 5 min. PCR products were separated by gel electrophoresis on 1.5% agarose gels and visualized by staining with ethidium bromide.
The PCR products were purified with the QIAquick PCR Purification Kit (Qiagen). The nucleotide sequences were determined by direct sequencing of the PCR products using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, California, USA) and analyzed on ABI PRISM
® DNA Analyzer (Applied Biosystems). The sequence data were aligned initially using the Clustal X program (version 1.8) [
16]. Additional sequences from representative anaplasmosis isolates were obtained from the GenBank database and then integrated with each set of alignments. A phylogenetic tree based on the nucleotide alignment was constructed using the neighbor-joining method [
17]. Bootstrap analysis was carried out with 1,000 replications and the tree was visualized using Tree-view.
Statistical analyses were performed by one-way ANOVA with IBM SPSS Statistics (version 19.0) and GraphPad Prism (version 6.0). In all statistical tests, P-value of <0.05 were considered significant.
Anaplasma infection was analyzed in a population of 39 Korean native goats; blood samples from 7 animals (17.9%) tested positive for
Anaplasma by 16S rRNA gene-based PCR. Infections with
Ehrlichia and
Rickettsia were not detected. None of the goats exhibited any clinical signs of illness, and no ticks were found on these animals. Additionally, no hematological abnormalities were observed. The incidence of
Anaplasma infection in Korean native goats in all age groups and in male and female goats was investigated.
Anaplasma infection was detected in female goats only (29.2%;
Table 1), and the incidence was 38.9% in goats of 12 months of age (
Table 2).
Anaplasma infection was not detected in male goats or in other age groups.
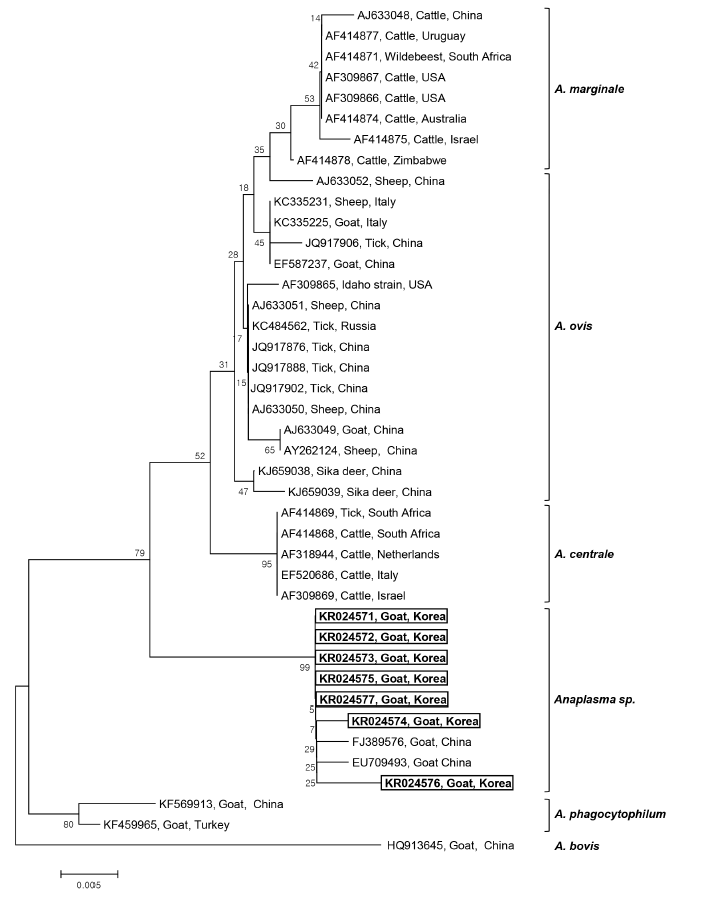
Seven 16S rRNA gene sequences of
Anaplasma sp. (GenBank Accession No. KR024571-KR024577) were obtained from the 7
Anaplasma-positive blood samples after direct sequencing. The sequence analysis of these 7 samples led to the identification of
Anaplasma sp. The phylogenetic tree analysis revealed that there were 3 main clusters for the established
Anaplasma spp.:
A. marginale,
A. ovis, and
A. centrale clusters. The 7 sequences obtained from Korean native goats on Jeju Island formed a fourth, separate cluster, that is,
Anaplasma sp., which diverged from
A. marginale,
A. centrale, and
A. ovis, but was closely related to
A. centrale (
Fig. 1). These isolates had a 98% similarity with
A. centrale, 96.4-97.5% similarity with
A. ovis, and 96.9-97.8% similarity with
A. marginale. The phylogenetic analyses showed that the 7
Anaplasma sp. identified in Korean native goats could potentially be new species, mainly because they clustered separately from the 3 established species,
A. marginale,
A. centrale, and
A. ovis. Our 7 sequences are quite similar to the species isolated from goats in China (EU709493 and FJ389576), which also belong to
Anaplasma sp., and are clustered in the same group. To our knowledge, this is the first study to identify
Anaplasma sp. infection in Korean native goats by PCR.
Although anaplasmosis is one of the most widespread tick-borne diseases, very little is known about
Anaplasma infection and its distribution in the ROK. Most studies concerning
Anaplasma in the ROK have addressed ticks and humans [
18,
19], while the role of
Anaplasma in goat pathology has not yet been described. In our study, a high prevalence of
Anaplasma infection was observed in female and adult goats although it was not statistically significant. This result indicates that adult goats may have more opportunities for exposure to ticks carrying the pathogen than younger animals. Additionally, our findings suggest the presence of
Anaplasma infection in Korean native goats on Jeju Island, which has a subtropical climate and a much higher distribution of ticks than other regions in the ROK. These conditions increase the possibility of tick-borne diseases spreading to livestock, wild animals, and humans.
In the present study, PCR and sequence analyses based on the 16S rRNA provided evidence of a new Anaplasma sp. infecting Korean native goats; these isolates were closely related to the previously reported Chinese isolates. The pathogenicity and role of Anaplasma sp. was not determined in Korean native goats; however, infection with this protozoan may have an impact on the health of these animals and consequently on their milk and meat production. Evidence of infection with a new Anaplasma sp. infection in Korean native goats would be very important, as the pathogens could considerably affect animal production, and outbreaks may occur under specific conditions. Accordingly, these findings indicate infection with a new species from the genus Anaplasma among Korean native goats on Jeju Island, ROK.
The current study demonstrates the presence of infection with Anaplasma sp. in Korean native goats, although none of the animals in our study exhibited clinical symptoms. Since the sample size was not sufficiently large to determine the incidence of Anaplasma infection, more studies are necessary to investigate epidemiological data and to elucidate the pathogenicity of Anaplasma sp. in these animals.
Notes
-
The authors declare that they have no competing interests.
This work was carried out with the support of "Cooperative Research Program for Agriculture Science & Technology Development (project no. PJ010092)", Rural Development Administration, the Republic of Korea.
Fig. 1.Phylogenetic analysis of 16S rRNA gene sequences derived from 7 Anaplasma-positive blood samples obtained from Korean native goats (Capra aegagrus hircus) on Jeju Island, ROK, together with previously registered sequences from Anaplasma species. The 16S rRNA gene sequences identified in the present study are shown in the black-lined box. The unrooted phylogenetic tree was constructed using the neighbor-joining methods. Bootstrapping was carried out with 1,000 replications.
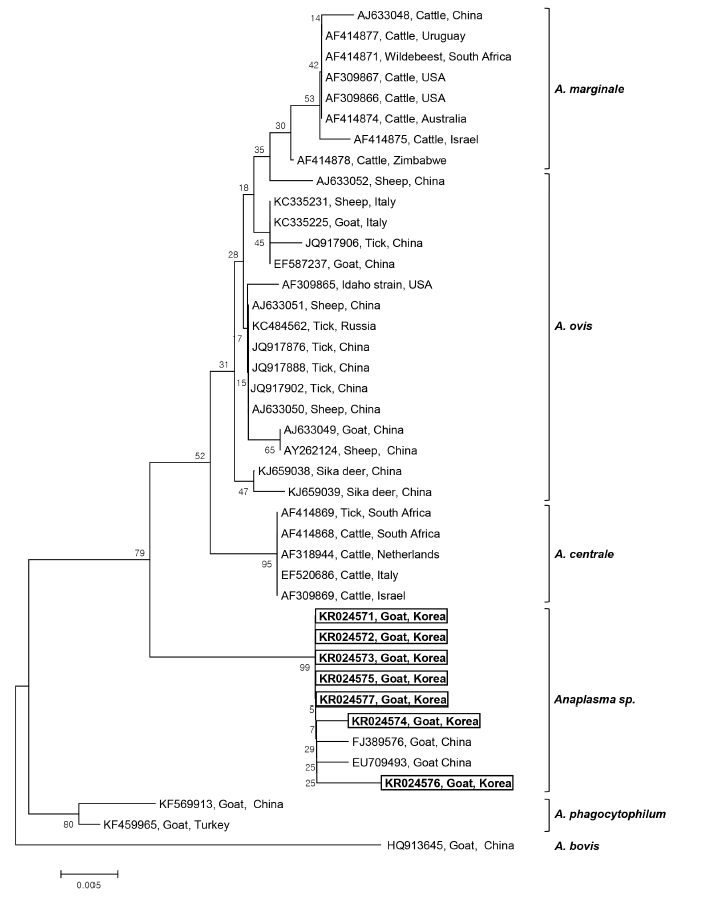
Table 1.Comparison of the number of Anaplasma-positive Korean native goats (Capra aegagrus hircus) by sex
Table 1.
|
Sex |
No. of goats |
No. of positive (%) |
|
Male |
15 |
0 (0.0) |
|
Female |
24 |
7 (29.2) |
|
Total |
39 |
7 (29.2) |
Table 2.Comparison of the number of Anaplasma-positive Korean native goats (Capra aegagrus hircus) by age
Table 2.
|
Age (months) |
No. of goats |
No. of positive (%) |
|
2 |
5 |
0 (0.0) |
|
3 |
5 |
0 (0.0) |
|
5 |
5 |
0 (0.0) |
|
10 |
5 |
0 (0.0) |
|
12 |
18 |
7 (38.9) |
|
24 |
1 |
0 (0.0) |
|
Total |
39 |
7 (38.9) |
References
- 1. Lew AE, Gale KR, Minchin CM, Shkap V, de Waal DT. Phylogenetic analysis of the erythrocytic Anaplasma species based on 16S rDNA and GroEL (HSP60) sequences of A. marginale, A. centrale, and A. ovis and the specific detection of A. centrale vaccine strain. Vet Microbiol 2003;92:145-160.
- 2. Torina A, Vicente J, Alongi A, Scimeca S, Turlá R, Nicosia S, Di Marco V, Caracappa S, de la Fuente J. Observed prevalence of tick-borne pathogens in domestic animals in Sicily, Italy during 2003-2005. Zoonoses Public Health 2007;54:8-15.
- 3. Inokuma H, Terada Y, Kamio T, Raoult D, Brouqui P. Analysis of the 16S rRNA gene sequence of Anaplasma centrale and its phylogenetic relatedness to other ehrlichiae. Clin Diagn Lab Immunol 2001;8:241-244.
- 4. Kocan KM, de la Fuente J, Blouin EF, Garcia-Garcia JC. Anaplasma marginale (Rickettsiales: Anaplasmataceae): recent advances in defining host-pathogen adaptations of a tick-borne rickettsia. Parasitology 2004;129:285-300.
- 5. Renneker S, Abdo J, Salih DE, Karagenç T, Bilgiç H, Torina A, Oliva AG, Campos J, Kullmann B, Ahmed J, Seitzer U. Can Anaplasma ovis in small ruminants be neglected any longer? Transbound Emerg Dis 2013;60:105-112.
- 6. Sreekumar C, Anandan R, Balasundaram S, Rajavelu G. Morphology and staining characteristics of Ehrlichia bovis. Comp Immunol Microbiol Infect Dis 1996;19:79-83.
- 7. Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, Grab DJ, Bakken JS. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis 2005;11:1828-1834.
- 8. Zhan L, Cao WC, Jiang JF, Zhang XA, Wu XM, Zhang WY, Liu W, Zuo SQ, Cao ZW, Yang H, Richardus JH, Habbema JD. Anaplasma phagocytophilum in livestock and small rodents. Vet Microbiol 2010;144:405-408.
- 9. Ramos RA, Latrofa MS, Giannelli A, Lacasella V, Campbell BE, Dantas-Torres F, Otranto D. Detection of Anaplasma platys in dogs and Rhipicephalus sanguineus group ticks by a quantitative real-time PCR. Vet Parasitol 2014;205:285-288.
- 10. Geurden T, Somers R, Thanh NT, Vien LV, Nga VT, Giang HH, Dorny P, Giao HK, Vercruysse J. Parasitic infections in dairy cattle around Hanoi, northern Vietnam. Vet Parasitol 2008;153:384-388.
- 11. Liu Z, Ma M, Wang Z, Wang J, Peng Y, Li Y, Guan G, Luo J, Yin H. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl Environ Microbiol 2012;78:464-470.
- 12. Kuttler KL. Anaplasma infections in wild and domestic ruminants: a review. J Wild Dis 1984;20:12-20.
- 13. Friedhoff KT. Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia 1997;39:99-109.
- 14. Baek BK, Jin CM, Seo SY, Seo YW, Kim DS, Kakoma I. A study on the epidemiology of caprine anaplasmosis in Korea. Korean J Vet Res 1994;34:381-386.
- 15. Lee SH, Jung BY, Kwak D. Evidence of Anaplasma spp. exposure in native Korean goats (Capra hircus coreanae). Vet Med 2015;60:248-252.
- 16. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 1997;25:4876-4882.
- 17. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 1987;4:406-425.
- 18. Kim CM, Kim MS, Park MS, Park JH, Chae JS. Identification of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and A. bovis in Haemaphysalis longicornis and Ixodes persulcatus ticks from Korea. Vector Borne Zoonotic Dis 2003;3:17-26.
- 19. Park JH, Heo EJ, Choi KS, Dumler JS, Chae JS. Detection of antibodies to Anaplasma phagocytophilum and Ehrlichia chaffeensis antigens in sera of Korean patients by western immunoblotting and indirect immunofluorescence assays. Clin Diagn Lab Immunol 2003;10:1059-1064.