Abstract
This study aimed to investigate the morphology and reconstruct the phylogenetic relationships of Centrocestus formosanus originating from 5 species of freshwater fish, i.e., Esomus metallicus, Puntius brevis, Anabas testudineus, Parambassis siamensis, and Carassius auratus, in Chiang Mai province, Thailand. Sequence-related amplified polymorphism (SRAP) and phylogeny based on internal transcribed spacer 2 (ITS2) and mitochondrial cytochrome c oxidase subunit 1 (CO1) were performed. The results showed similar morphologies of adult C. formosanus from day 5 after infection in chicks. C. formosanus originated from 4 species of freshwater fish had the same number of circumoral spines on the oral sucker, except for those from C. auratus which revealed 34 circumoral spines. The phylogenetic tree obtained from SRAP profile and the combination of ITS2 and CO1 sequence showed similar results that were correlated with the number of circumoral spines in adult worms. Genetic variability of C. formosanus also occurred in different species of freshwater fish hosts. However, more details of adult worm morphologies and more sensitive genetic markers are needed to confirm the species validity of C. formosanus with 34 circumoral spines originating from C. auratus in the future.
-
Key words: Centrocestus formosanus, molecular phylogeny, freshwater fish, Chiang Mai, Thailand
INTRODUCTION
Centrocestus formosanus (Digenea: Heterophyidae) is a small intestinal trematode of fish-eating birds and mammals, including ducklings, chickens, rats, rabbits, cats, dogs, and foxes [
1]. The worm was reported to be infected in humans in Taiwan, Japan, and Lao PDR [
2,
3]. It is distributed in many countries, i.e., Taiwan (China), Japan, the Philippines, India, Lao PDR, Mexico, and Brazil [
3–
5].
Centrocestus caninus is a closely related species to C. formosanus. It has been reported to infect humans in Thailand and Lao PDR [
6,
7]. Various species of freshwater fish act as the second intermediate host of C. formosanus, including
Puntius brevis,
Cyclocheilichthys repasson, Osteochilus hasseltii, and
Australoheros facetus [
2,
5]. In northern Thailand,
Centrocestus metacercariae have been highly infected in freshwater fish, including
P. brevis,
Esomus metallicus, and
Anabas testudineus [
8–
10]. Infection can lead to a reduction in respiratory capacity and cause fish mortality [
11].
Encysted metacercariae are oval-shaped, and the larvae present an X-shaped excretory bladder. Excysted metacercariae are pyriform-shaped and have a terminal oral sucker equipped with 2 alternate rows of 32 circumoral spines. The adult stage is similar to its excysted metacerariae, except for the presence of eggs within the uterus. The number of circumoral spines is the main character for distinguishing the species belonging to the genus
Centrocestus [
1]. They are classified into 3 groups, i.e., those with 26–36 spines (
C. formosanus, C. yokogawai, C. caninus, C. longus, C. cuspidatus, and
C. asadai), 38–48 spines (
C. nycticoracis, C. kurokawai, and
C. armatus), and 50–60 spines (
C. polyspinosus) [
6]. Among the first group, the shape of the excretory bladder is adopted as a useful key.
C. cuspidatus has a V-shaped excretory bladder, and the remaining 5 species have an X-shaped excretory bladder [
6]. However, their morphology is similar particularly in their larval stages, and counting the exact number of circumoral spines is difficult in some specimens. So, it is difficult to identify the species by standard methods.
Currently, molecular approaches are one of the most effective and accurate methods for genetic characterization of helminths. Sequence-related amplified polymorphism (SRAP) is an efficient genetic marker system, revealing genetic variation in open reading frames among related organisms [
12]. It has been used to study genetic variation in some parasites such as the cattle liver fluke,
Fasciola hepatica [
13]. However, there are many DNA regions that are useful for identification of helminths, discriminating between species with similar morphologies, and useful for phylogenetic studies, especially the internal transcribed spacer 2 (ITS2) in nuclear genes and the mitochondrial cytochrome c oxidase subunit 1 (CO1) in mitochondrial genes [
14,
15]. For molecular identification and phylogeny of
Centrocestus, Mehrdana et al. [
16] reported that
Centrocestus metacercariae found in fish,
Xiphophorus malculatus, imported from Singapore revealed the highest identity (> 99%) with
Centrocestus sp. originating from Iran based on ITS2 region. Chai et al. [
3] reported that 18S rRNA was used to identify
C. formosanus isolated from humans in Lao PDR, which showed 100% nucleotide identity with the U.S. isolate [
3]. Later, Chontananarth et al. [
14] studied the phylogeny of heterphyid flukes based on CO1 gene and reported that
C. caninus was closely related to
Haplorchoides sp. and
Pygidiopsis summa. However, it has never been reported about the studies of
C. formosanus relationships in difference hosts before.
So, this work aimed to study the morphological details and phylogenetic relationships of adult C. formosanus obtained from 5 species of freshwater fish in Chiang Mai province, Thailand by using SRAP PCR and phylogeny based on ITS2 and mCO1 regions.
MATERIALS AND METHODS
Worm sample preparation
C. formosanus metacercariae were collected from 5 freshwater fish species; flying barb (Esomus metallicus), golden little barb (Puntius brevis), climbing perch (Anabas testudineus), longfin mojarra (Parambassis siamensis), and crucian carp (Carassius auratus) in Mae Tang, San Sai, and Meuang district, Chiang Mai province, Thailand.
Seven trematode species at the adult stage were prepared from the metacercarial stage: Centrocestus from 5 freshwater fish, Stellantchasmus falcatus from green backed (Mugil dussumieri), and Stellantchasmus sp. from wrestling half-beak (Dermogynus pusillus). All metacercariae were force-fed to the experimental host (1-day-old chick). For the other parasites, adult worms of Haplorchis taichui were collected from humans, Haplorchoides sp. from yellow cat fish (Hemibagus filamentus), and the giant liver fluke, Fasciola gigantica, was obtained from a cow (Bos tauras). All specimens were rinsed several times with tap water and then frozen at −20°C immediately for DNA extraction.
Morphological observation of Centrocestus formosanus adult
C. formosanus adults were obtained from chicks 5 days after infection, fixed in 4% formalin, slightly flattened under a coverslip, and prepared for permanent slides. All permanent slides were photographed and then identified based on Yamaguti [
17].
DNA extraction
Genomic DNA was extracted by using Chelex solution. In brief, 150 μl of 5% Chelex (Fluka) solution containing 10 μl of proteinase K (Sigma, Poole, UK) at a concentration of 20 mg/ml was added to approximately 20 mg of the trematode tissue. It was then heated at 55°C for 1 hr, followed by gentle vortexing and heating at 95°C for 30 min, again followed by gentle vortexing. The mixture was centrifuged at 13,000 g for 1 min. The supernatant was collected and stored at −20°C until it was used.
SRAP PCR
Ten different primer combinations (5 forward primers and 5 reverse primers) were used to perform DNA fingerprints from different species of adult parasites. SRAP PCR reaction was carried out in a final volume of 20 μl. The reactions were performed in a Thermal Cycler machine (Little Genius, Bioer Technology, Minato-ku, Tokyo, Japan). PCR protocols were indicated as follows: 5 min of initial denaturation at 94°C, followed by 10 cycles of 3 steps: 1 min of denaturation at 94°C, 1 min of annealing at 35°C, 1 min of extention at 72°C, followed by 35 cycles with annealing temperature being increased to 50°C, with a final extension at 72°C for 5 min. PCR products were separated on 1.4% TBE agarose gel electrophoresis stained with DNA-Dye NonTox (Applichem, Darmstadt, Germany) and photographed with a Sony digital camera (DSC-WX50).
Amplification of ITS2 region and CO1 gene
The ITS2 regions were amplified by using the primers, forward 3S (5′-GGT ACC GGT GGA TCA CTC GGC TCG TG-3′) and reverse BD2 (5′-TAT GCT TAA ATT CAG CGG GT-3′). The PCR conditions were as follows: 2 min initial denaturation at 94°C, followed by 35 cycles of 1 min DNA denaturation at 94°C, 1 min primer annealing at 57°C, and 1 min extension at 72°C, and a final extension at 72°C for 7 min.
The CO1 genes were amplified by using the primers, JB3 (5′-TTT TTT GGG CAT CCT GAC GTT TAT-3′) as the forward primer and JB4.5 (5′-TAA AGA AAG AAC ATA ATG AAA ATG-3′) as the reverse primer. The PCR conditions were as follows: 3 min initial denaturation at 95°C, followed by 40 cycles of 1 min DNA denaturation at 95°C, 1 min primer annealing at 50°C, 1 min extension at 72°C, and a final extension at 72°C for 7 min.
Agarose gel electrophoresis with DNA–Dye NonTox staining was used to visualize ITS2 and CO1 products under UV light. All ITS2 and CO1 PCR products were purified and subjected to sequencing.
Data analysis
SRAP fragments were scored for presence (the presence of the specific allele, code “1”) or absence (the absence of the specific allele, code “0”) in each sample. The distance matrix and dendrogram were constructed using phylogeny inference package (PHYLIP) version 3.695. A phylogenetic tree was constructed based on the combined data set of all primers, using the unweighted pair group method with arithmetic averages (UPGMA).
For the ITS2 and CO1 sequences, the sequences were aligned using ClustalW, then both genes were combined together. The phylogenetic tree was reconstructed using MEGA version 6.0 based on neighbor-joining (NJ) and maximum-likelihood (ML) methods. The statistics supported for the branches were tested using 1,000 bootstrap replicates.
RESULTS
Morphological characteristics of Centrocestus formosanus adults
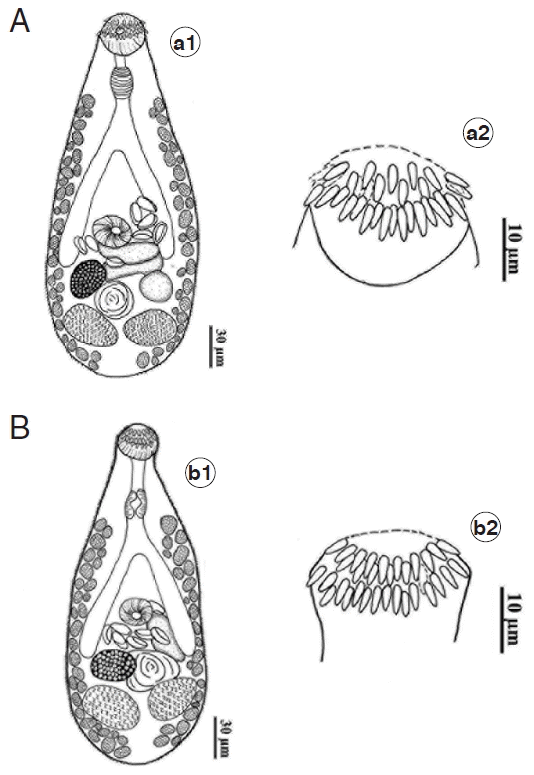
C. formosanus obtained from the chick, 5 days after infection with metacercarial stage from 5 species of freshwater fish, were mostly similar in their morphology with the original description of this species (
Fig. 1A, B). It can be described as body pyriform-shaped with broad posterior half and covered with scale-like tegumental spines from the anterior to the posterior end. Prepharynx present. Pharynx well developed. Esophagus short. Ceca large, bifurcated about midway between oral and ventral suckers, terminated slightly in front of ovary. Ventral sucker smaller than oral sucker and located in the middle of body. Ovary oval, located on right side of posterior half of body. Testes oval, opposite in posterior body. Eggs oval, operculum distinct. Excretory bladder X-shaped.
There was only 1 character of
C. formosanus that showed difference between adult flukes originated from 5 species of fish. That is the number of circumoral spines surrounding the oral sucker of C. formosanus. The adults originated from
C. auratus differed from the worms originated from the 4 remaining fish species. Those from 4 freshwater fish species (
Esomus metallicus, Puntius brevis, Anabas testudineus, and
Parambassis siamensis) had their oral sucker with 2 alternate rows of 32 circumoral spines (
Fig. 1A); however, 34 circumoral spines were found in worms from
C. auratus (
Fig. 1B). The measurements of
C. formosanus isolated from 5 species of fish were varying and overlapped. Their measurements are shown in
Table 1.
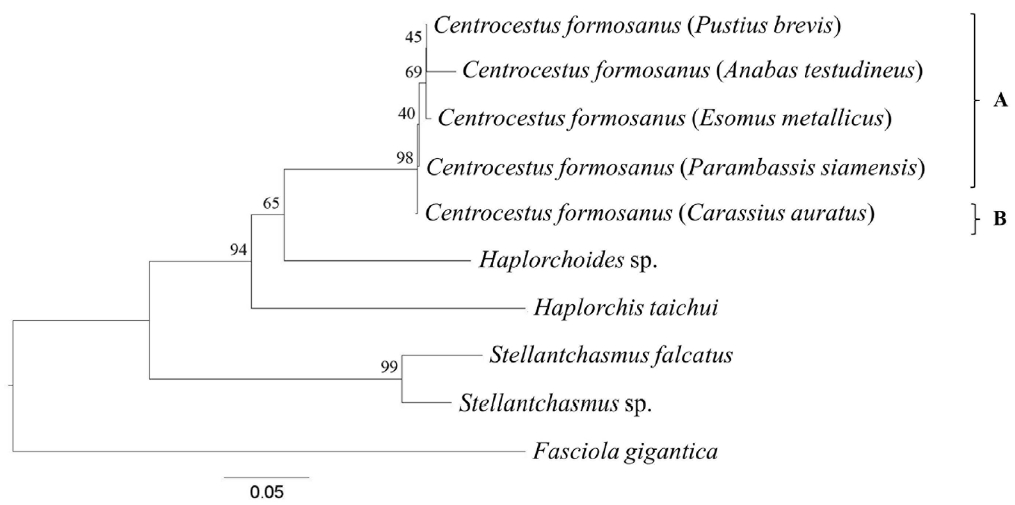
Ten SRAP primer combinations showed better polymorphisms of 10 representative trematodes: ME1/EM1, ME2/EM3, ME3/EM4, ME4/EM5, ME1/EM3, ME2/EM1, ME4/EM6, ME5/EM4, ME3/EM6, and ME4/EM1. These primers were chosen for phylogenetic relationship construction. UPGMA phylogram analysis based on SRAP profile revealed a monophyletic group of
C. formosanus. They were divided into 2 main groups. The first group was
C. formosanus originating from
E. metallicus,
P. brevis,
A. testudineus, and
P. siamensis (clade A,
Fig. 2). The second one was those isolated from
C. auratus (clade B,
Fig. 2).
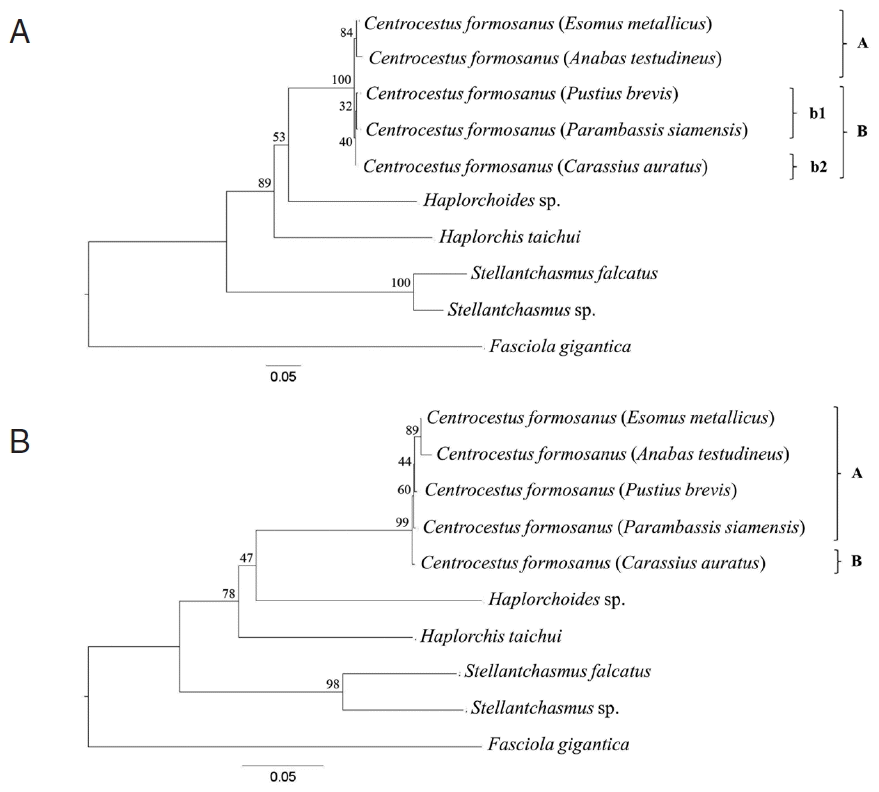
Phylogenetic trees were reconstructed based on a character method (ML) and a distance method (NJ) with bootstrap values of 1,000 replicates. Both methods showed the similar topology that the
Centrocestus group was monophyly, as shown in
Fig. 3. In ML tree,
C. formosanus was separated into 2 groups:
C. formosanus originating from
E. metallicus and
A. testudineus (clade A,
Fig. 3A), and
C. formosanus originating from
P. brevis,
P. siamensis, and
C. auratus (clade B,
Fig. 3A) with low bootstrap values. In NJ tree,
C. formosanus isolated from
C. auratus was separated from the 4 remaining specimens with low bootstrap values (clade B,
Fig. 3B).
DISCUSSION
In the present study, 5 species of freshwater fish, i.e.,
E. metallicus,
P. brevis, A. testudineus,
P. siamensis, and
C. auratus were highly infected with
Centrocestus metacercariae. The adult worms from chicks 5 days after infection were mostly similar in their morphology having 32 circumoral spines except for the host
C. auratus from which the worm had 34 circumoral spines. The number of circumoral spines of
C. formosanus is 32 spines in most cases [
1,
18]. It is also similar to a previous study that reported 32 circumoral spines of
C. formosanus [
3–
5]. This species differed from the related species;
C. caninus which has 26–30 spines [
6]. They were also different in the presence of a prepharynx in
C. formosanus, whereas
C. caninus has no prepharynx [
6]. The number of circumoral spines was accepted as the most reliable criteria although the number varied showing a range in some species [
1]. However, it has some doubts that
C. formosanus originating from
C. auratus revealed 34 circumoral spines in all specimens. It is different from other studies which reported 32 spines in most cases.
Only a few studies have demonstrated the molecular characterization of heterophyid trematodes, i.e.,
Centrocestus, recovered in freshwater fish in Thailand, especially in the northern region [
14,
19]. The molecular approach and DNA sequencing techniques have been successfully developed for identification and studies of their phylogenetic relationships. In our study, 2 molecular techniques (SRAP PCR and phylogeny) and 2 DNA regions (ITS2 and CO1 regions) were performed for studying the phylogenetic relationships among
C. formosanus recovered from different fish species. Our analysis revealed a monophyletic tree of
C. formosanus. It was separated from other heterophyid trematodes and out group (
F. gigantica) with moderate bootstrap values (
Figs. 2,
3).
The trees were derived based on SRAP profile and ITS2 and CO1 sequences. All tree topologies were similar to each other (
Figs. 2,
3). However, the tree from concatenate data set revealed some difference topology with the individual gene trees. Within the
C. formosanus group, there were 2 main clades which had the same character as the circumoral spine numbers (clades ‘A’ and ‘B’;
Figs. 2,
3B).
C. formosanus with 34 spines originated from
C. auratus (clade ‘B’;
Figs. 2,
3B) was separated from
C. formosanus with 32 spines (clade ‘A’;
Figs. 2,
3B). However,
C. formosanus originated from
C. auratus were also divided into a subclade (clade ‘b2’;
Fig. 3A) that is associated with the number of circumoral spines that were divided into 32 and 34 spine groups. Although,
C. formosanus isolated from
C. auratus (clade ‘b2’;
Fig. 3A) was closely related to
C. formosanus isolated from
P. brevis and
P. siamensis (clade ‘b1’;
Fig. 3A).
In our study, 5 species of freshwater fish are divided into 3 families, including Cyprinidae, Anabantidae, and Ambassidae.
C. formosanus isolated from
E. metallicus (Cyprinidae) and
A. testudineus (Anabantidae) were grouped into the same group (clade ‘A’;
Fig. 3A), whereas those from
P. brevis (Cyprinidae) and
P. siamensis (Ambassidae) were grouped together (clade ‘B’;
Fig. 3A). However, the worms from
C. auratus (Cyprinidae) were separated into other group (clade ‘b2’;
Fig. 3A). These results agreed with the reason that intraspecific variation among specimens from different host group was low because of their functional constraints [
13,
20]. However, they have genetic variability within the same host group of the family Cyprinidae. For the genetic divergence of both genes, ITS2 and CO1 revealed that the distance value between
C. formosanus with 32 and 34 spines were 0.007 (0.7%) and 0.025 (2.5%), respectively. These indicated that both
C. formosanus groups may be different which correlated to the number of circumoral spines.
In conclusion, our study demonstrated that C. formosanus originating from 5 freshwater fish species were morphologically similar, except for a difference in the number of circumoral spines (32 and 34 spines). Molecular techniques were a useful tool for the study of phylogenetic relationships among C. formosanus originating from difference species of freshwater fish; the worms originated from C. auratus were genetically unique from those originated from other 4 species of freshwater fish. However, more sensitive genetic markers are needed to confirm the species validity of a new genotype, i.e., C. formosanus having 34 circumoral spines originating from C. auratus.
Notes
-
CONFLICT OF INTEREST
We have no conflict of interest related to this work.
ACKNOWLEDGMENTS
We would like to thank the staff of the Applied Parasitology Research Laboratory, Department of Biology, Faculty of Science, Chiang Mai University for use of their facilities, and the Science and Technology Research Institute, Chiang Mai University for financial support. Special thanks are extended to Mr. Nathaniel S. Kimmons for manuscript correction.
Fig. 1The morphology of adult Centrocestus formosanus with 32 and 34 circumoral spines. (A) C. formosanus originated from Puntius brevis (a1) showing 32 spines arranged in 2 alternate rows around its oral sucker (a2). (B) C. formosanus originated from Carassius auratus (b1) showing 34 spines arranged in 2 alternate rows around its oral sucker (b2).

Fig. 2Cladogram constructed based on SRAP profile using UPGMA method. The adult worms of clade ‘A’ had 32 circumoral spines, whereas those of clade ‘B’ had 34 circumoral spines.
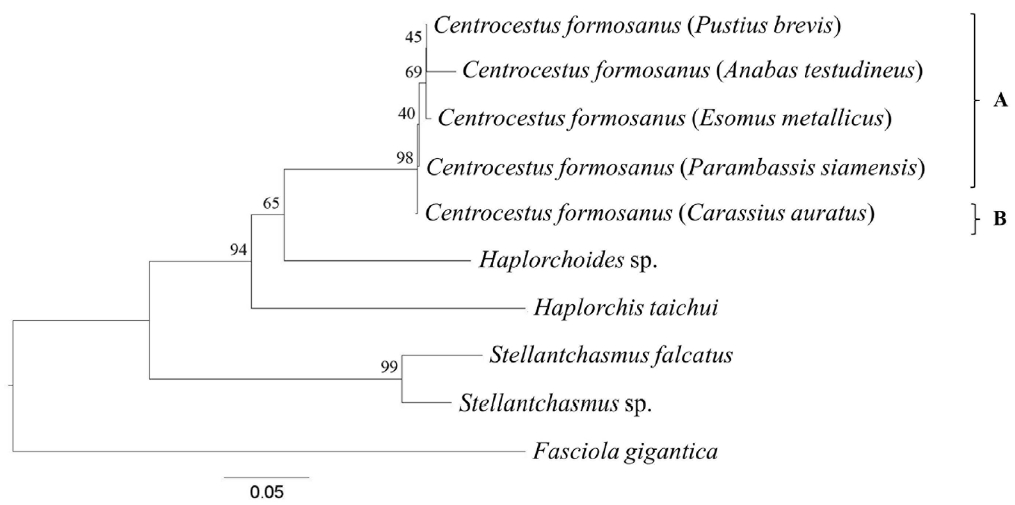
Fig. 3Cladogram constructed based on the combination of ITS2 and CO1 sequence with 1,000 bootstrap replicates. (A) Maximum-likelihood (ML) method. The adult worms of clade ‘A’ and clade ‘B b1’ had 32 circumoral spines, whereas those of clade ‘B b2’ had 34 circumoral spines. (B) Neighbor-joining (NJ) method. The adult worms of clade ‘A’ had 32 circumoral spines, whereas those of clade ‘B’ had 34 circumoral spines.
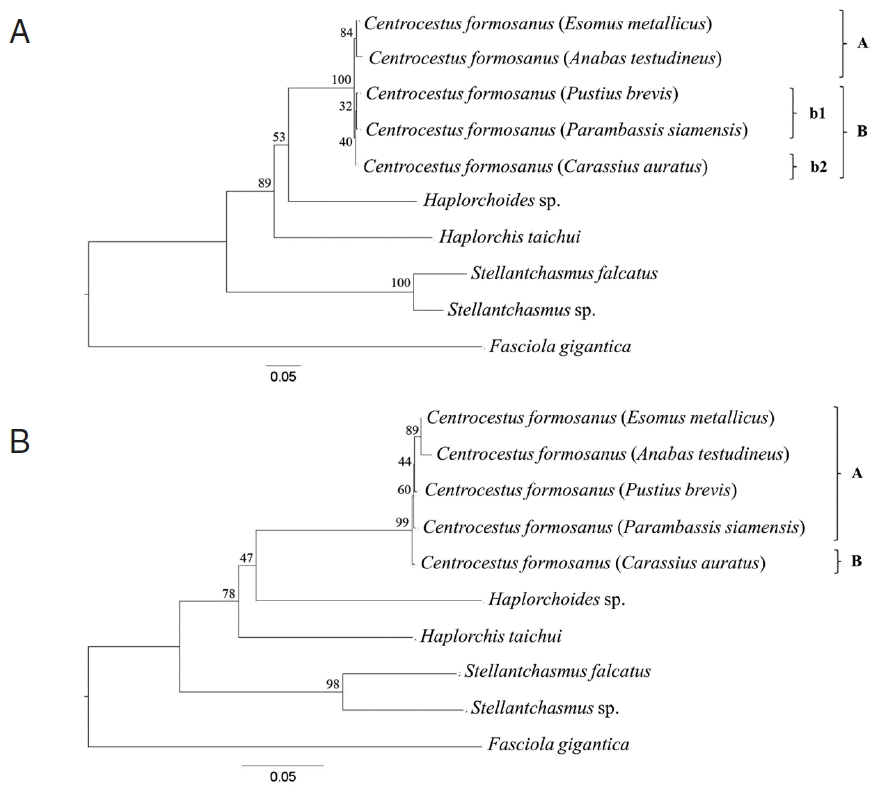
Table 1Measurements of Centrocestus formosanus adults recovered in the small intestines of chicks at day 5 post infection
Table 1
|
Esomus metallicus (n=5) |
Puntius brevis (n=5) |
Anabas testudineus (n=5) |
Parambassis siamensis (n=5) |
Carassius auratus (n=5) |
|
No. of oral spines |
|
32 |
32 |
32 |
32 |
34 |
|
|
Body |
L |
610–750 (670) |
590–710 (632) |
540–900 (660) |
432–640 (537.3) |
600–750 (668) |
|
W |
270–350 (298) |
240–320 (276) |
220–290 (252) |
196–270 (237) |
200–290 (259) |
|
|
Oral sucker |
L |
57.5–72.5 (66.5) |
57.5–80 (69.5) |
55–65 (61.5) |
42–65 (72.5) |
60–80 (70) |
|
W |
62.5–87.5 (78.5) |
65–80 (72.5) |
65–82.5 (72.5) |
58–72.5 (64.3) |
52.5–82.5 (76) |
|
|
Ventral sucker |
L |
50–75 (60) |
47.5–60 (54) |
50–62.5 (56.9) |
44–55 (49.7) |
52.5–57.5 (55.5) |
|
W |
62.5–70 (64) |
52.5–62.5 (58) |
55–72.5 (61.9) |
50–62.5 (58.3) |
55–70 (65.5) |
|
|
Prepharynx |
|
17.5–82.5 (53) |
35–50 (44) |
7.5–62.5 (36) |
22–82.5 (44) |
17.5–32.5 (26.5) |
|
|
Pharynx |
L |
37.5–62.5 (51.5) |
35–62.5 (47) |
45–55 (51) |
40–50 (46.7) |
45–57.5 (49.5) |
|
W |
37.5–52.5 (47) |
35–45 (40.5) |
25–50 (40) |
32–52.5 (41.5) |
37.5–50 (44) |
|
|
Esophagus |
L |
50–100 (64.5) |
30–80 (54.5) |
30–62.5 (45) |
20–50 (31.7) |
32.5–62.5 (51.5) |
|
|
Ovary |
L |
67.5–100 (82) |
55–90 (79) |
50–100 (71.9) |
40–67.5 (52.5) |
62.5–87.5 (76.5) |
|
W |
62.5–137.5 (103.5) |
70–150 (99.5) |
75–125 (96.9) |
66–82.5 (73.3) |
65–150 (105.5) |
|
|
Right testis |
L |
100–120 (110.5) |
70–100 (85) |
65–120 (85) |
68–100 (84.3) |
57.5–92.5 (76) |
|
W |
62.5–157.5 (121) |
92.5–155 (119.5) |
82.5–112.5 (96.25) |
88–125 (107.7) |
100–137.5 (120.5) |
|
|
Left testis |
L |
87.5–137.5 (103.5) |
75–100 (84.5) |
62.5–87.5 (71.9) |
60–87.5 (74.2) |
67.5–125 (87) |
|
W |
50–142.5 (108.5) |
87.5–120 (87.5) |
87.5–105 (95) |
80–120 (100) |
82.5–125 (103.5) |
|
|
Egg |
L |
32.5–40 (38) |
37.5–40 (39) |
37.5–42.5 (41) |
34–42.5 (38.8) |
40–47.5 (43.5) |
|
W |
22.5–22.5 (22.5) |
17.5–25 (20.5) |
20–22.5 (21) |
20–25 (22.5) |
20–20 (20) |
References
- 1. Chen HT. The metacercaria and adult of Centrocestus formosanus (Nishigori, 1924) with notes on the natural infection of rats and cats with C. armatus (Tanabe, 1922). J Parasitol 1942;28:285-298.
- 2. Chai JY, Shin EH, Lee SH, Rim HJ. Foodborne intestinal flukes in Southeast Asia. Korean J Parasitol 2009;47( suppl):69-102.
- 3. Chai JY, Sohn WM, Yong TS, Eom KS, Duk-Young Min DY, Lee MY, Lim H, Insisiengmay B, Phommasack B, Rim HJ. Centrocestus formosanus (Heterophyidae): human infection and the infection source in Lao PDR. J Parasitol 2013;99:531-536.
- 4. Han ET, Shin EH, Phommakorn S, Sengvilaykham B, Kim JL, Rim HJ, Chai JY. Centrocestus formosanus (Digenea: Heterophyidae) encysted in the freshwater fish, Puntius brevis from Lao PDR. Korean J Parasitol 2008;46:49-53.
- 5. Pinto HA, Melo AL. Metacercariae of Centrocestus formosanus (Trematoda: Heterophyidae) in Australoheros facetus (Pisces: Cichlidae) in Brazil. Rev Bras Parasitol Vet 2012;21:334-337.
- 6. Waikagul J, Wongsaroj T, Radomyos P, Meesomboon V, Praewanich R, Jongsuksuntikul P. Human infection of Centrocestus caninus in Thailand. Southeast Asian J Trop Med Public Health 1997;28:831-835.
- 7. Chai JY, Park JH, Han ET, Guk SM, Shin EH, Kim JL, Sohn WM, Yong TS, Eom KS, Min DY, Huang EH, Phommmasack B, Insisiengmay B, Rim HJ. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane Municipality and Saravane Province in Laos. J Helminthol 2005;79:283-289.
- 8. Phalee A, Wongsawad C, Wongsawad P, Chuboon S. The infection rate of Centrocestus caninus (Leiper, 1913) in Swamp barb, Puntius brevis (Bleeker, 1860) from Mae Taeng district, Chiang Mai province. J Yala Rajabhat Univ 2009;4:92-99.
- 9. Noikong W, Wongsawad C, Phalee A. Seasonal variation of metacercariae in cyprinoid fish from Kwae Noi Bamroongdan Dam, Phitsanulok province, northern Thailand. Southeast Asian J Trop Med Public Health 2011;42:58-62.
- 10. Luangphai P, Wongsawad C, Kumchoo K, Sripalwit P. Survey of helminthes in climbing perch (Anabas testudineus) from San Sai district, Chiang Mai province. Southeast Asian J Trop Med Public Health 2004;35:288-290.
- 11. Mitchell AJ, Overstreet RM, Goodwin AE, Brandt TM. Spread of an exotic fish-gill tremetode: a far-reaching and complex problem. Fisheries 2005;30:11-16.
- 12. Li G, Quiros CF. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: its application to mapping and gene tagging in Brassica. Theor Appl Genet 2001;103:455-461.
- 13. Alasaad S, Li QY, Lin RQ, Martín-Atance P, Granados JE, Díez-Baños P, Pérez JM, Zhu XQ. Genetic variability among Fasciola hepatica samples from different host species and geographical localities in Spain revealed by the novel SRAP marker. Parasitol Res 2008;103:181-186.
- 14. Chontananarth T, Wongsawad C, Chomdej S, Krailas D, Chai JY. Molecular phylogeny of trematodes in family Heterophyidae based on mitochondrial cytochrome c oxidase subunit 1 (mCO1). Asian Pac J Trop Med 2014;7:446-450.
- 15. Sripalwit P, Wongsawad C, Chontananarth T, Anuntalabhochai S, Wongsawad P, Chai JY. Developmental and phylogenetic characteristics of Stellantchasmus falcatus (Trematode: Heterophyidae) from Thailand. Korean J Parasitol 2015;53:201-207.
- 16. Mehrdana F, Jensen HM, Kania PW, Buchmann K. Import of exotic and zoonotic trematodes (Heterophyidae: Centrocestus sp.) in Xiphophorus malculatus: implications for ornamental fish import control in Europe. Acta Parasitol 2014;59:276-283.
- 17. Yamaguti S. Digenetic Trematodes of Vertebrates. In Yamaguti S ed, Systema Helminthum. New York, USA. Interscience; 1958, pp 706-707.
- 18. Premvati A, Pande V. On Centrocestus formosanus (Nishigori, 1924) Price, 1932 and its experimental infection in white leghorn chicks. Jpn J Parasitol 1974;23:79-84.
- 19. Wongsawad C, Wongsawad P, Anuntalabhochai S, Chai JY, Sukontason K. Occurrence and molecular identification of liver and minute intestinal flukes metacercariae in freshwater fish from Fang-Mae Ai agricultural basin, Chiang Mai province, Thailand. Asian Biomed (Res Rev News) 2013;7:97-104.
- 20. Crow JF. The origins, patterns and implications of human spontaneous mutation. Nat Rev Genet 2000;1:40-47.