Abstract
Fascioliasis is a parasitic infection caused by liver flukes. Although several cases have been reported in Korea, phylogenetic analysis of isolates is lacking. In this study, a 66-year-old woman with right upper quadrant (RUQ) abdominal pain was diagnosed as fascioliasis involving abdominal muscle by imaging study. She received praziquantel treatment, but symptoms were not improved. Lateral movement of the abscess lesion was followed. Trematode parasite was surgically removed from the patient’s rectus abdominis muscle. The fluke was identified as Fasciola hepatica based on sequence analysis of 18S rDNA. To determine the phylogenetic position of this Fasciola strain (named Korean Fasciola 1; KF1), the cox1 gene (273 bp) was analyzed and compared with the genes of 17 F. hepatica strains isolated from cows, sheep, goats, and humans from various countries. Phylogenetic analysis showed that KF1 was closely related with the isolates from China goat.
-
Key words: Fasciola hepatica, genetic analysis, phylogenetic
Fascioliasis in livestock and humans are caused by the genus
Fasciola [
1].
Fasciola spp. are prevalent in temperate climates. Fascioliasis is one of the neglected zoonotic diseases declared by the World Health Organization (WHO) [
2]. Incidence of fascioliasis has been significantly increased since 1980, affecting approximately 2.4 million people globally each year. In humans, infection occurs through ingestion of contaminated water containing
Fasciola metacercaria(e). The larvae pass through the stomach, break through the duodenal barrier, penetrate the abdominal cavity, and enter the hepatobiliary system. When the ingested fluke moves to the bile duct through the abdominal cavity and liver parenchyma, extensive bleeding and inflammation occur, causing thickening and expansion of the bile duct and gall bladder [
3,
4]. The symptoms and signs of human fascioliasis occur in 2 stages. The hepatic stage of the disease occurs when juvenile
Fasciola enters the liver and begins to migrate to the biliary root canal; this is referred to as the acute or invasive stage. This stage occurs 1–3 months after the ingestion of metacercariae, in which typical symptoms and signs include fever, hives, hepatomegaly, and pain in the right hypochondrium. When the worm infects biliary tract, cholangitis, cholestasis, right upper quadrant (RUQ) pain, and eosinophilia are usually developed [
5–
7].
Genetic diversity of
Fasciola populations has been demonstrated via molecular analysis of their nuclear and mitochondrial DNAs (mtDNAs). The polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis of mtDNA revealed 52 unique sequences in 221 flukes [
8]. A study on
Fasciola isolates from livers of sheep and cattle suggested grouping of these isolates into 3 main lineages (
F. hepatica, F. gigantica, and
F. indica), using the
cox1 (encoding cytochrome c oxidase subunit) gene. Ai et al. [
1] also inferred phylogeny using the
cox1, which is a useful marker for studying the genetic differentiation and phylogenetic relationship of
Fasciola.
Infection with
Fasciola spp. has been reported in East Asia, including Korea (
Table 1) and Japan, while few molecular taxonomic studies have been conducted on Korean species [
10]. In this study, the genetic characteristics of
Fasciola isolated from a Korean patient were compared with those of several previously reported isolates using the
cox1 gene sequencing and multiple alignment methods.
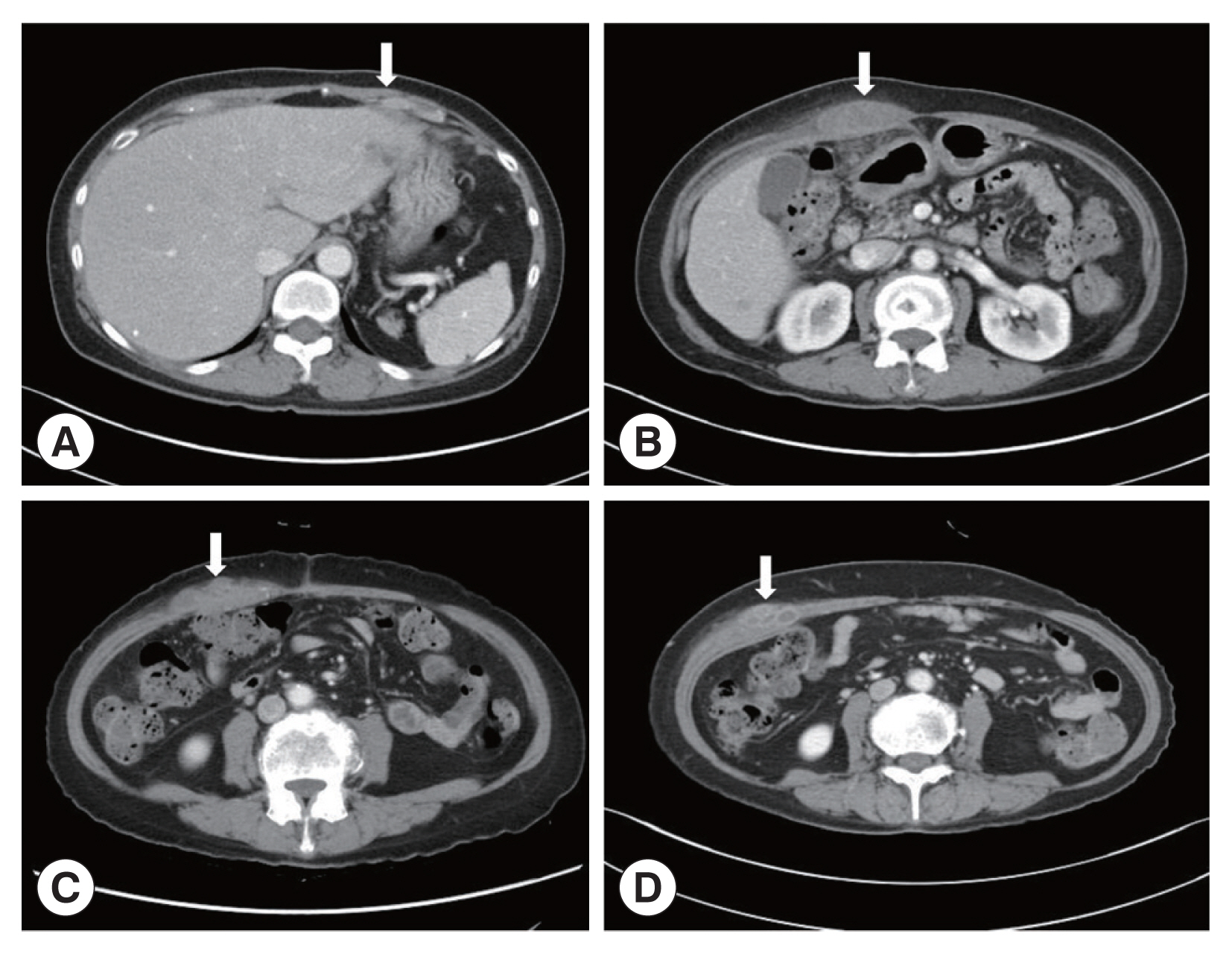
A 66-year-old Korean woman living in Pusan visited the Pusan National University Hospital on July 11, 2021, with an abdominal mass. She had been suffered from hypothyroidism. In June 2021, she was diagnosed with a hepatic abscess by computed tomography (CT) performed during an evaluation of RUQ pain at another hospital. The symptoms improved by symptomatic treatment and she was discharged after administration of antibiotics (ceftriaxone and metronidazole) (
Fig. 1A). She visited again our hospital due to recurrence of the RUQ pain. The mass was palpable in the abdominal cavity, we checked the mas with CT. Some swelling and cell infiltration was confirmed, and parasitic infection was suspected (
Fig. 1B). She received praziquantel (25 mg/kg 3 days). Most fascioliasis patients showed eosinophilia and abdominal distension [
11]. Abdominal distension along with eosinophilia was also confirmed in this patient. In August 2021, liver abscess was percutaneously drained, after which she was discharged. However, she complained of new pain in the subcostal region (
Fig. 1C). Praziquantel treatment were maintained, but her symptoms did not improve. New multiple abscesses were found below the previous lesion on abdominal CT. Compared to the previous CT, a lateral movement of the abscess was observed with maximum size of 3.4 cm (
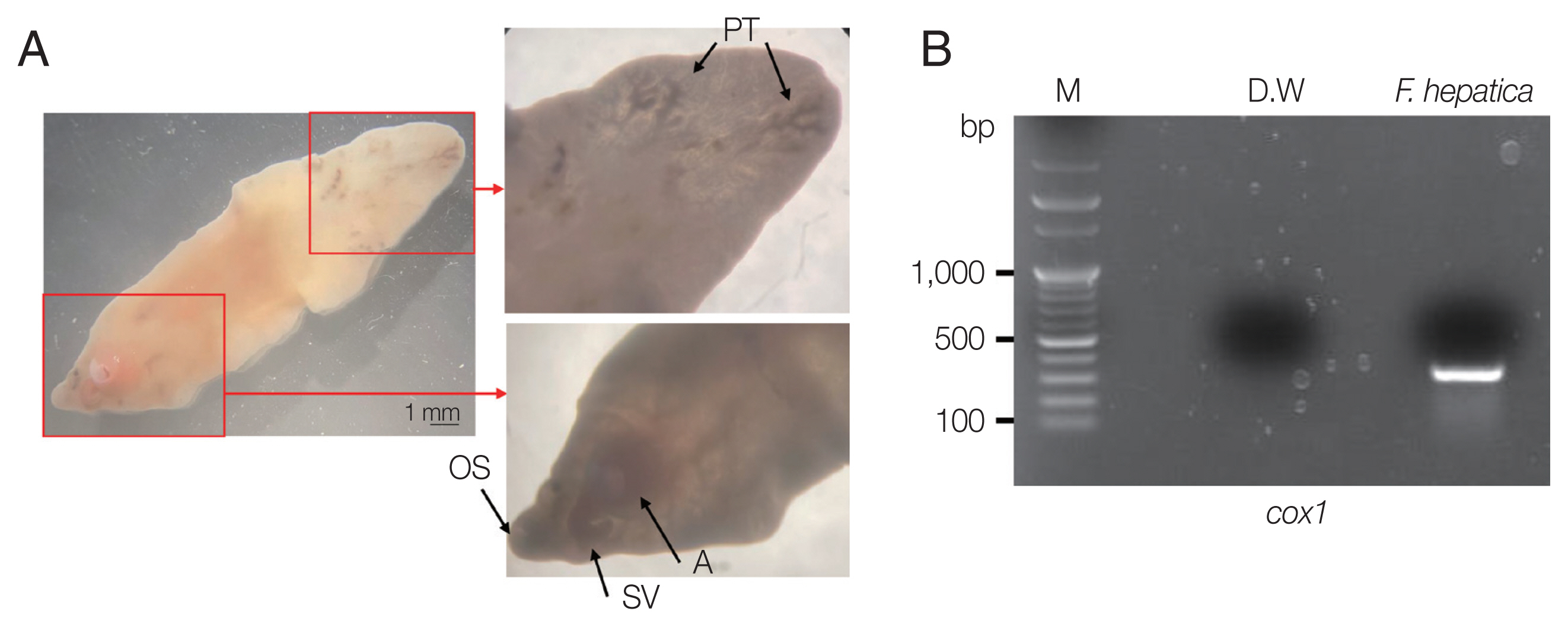
Fig. 1D). The CT showed abdominal distension. The laboratory test showed eosinophilia (7.3%, normal range 0–6.9%) with low number of segmented neutrophil (38.8%, normal range 40.0–70.4%). The mean corpuscular hemoglobin concentration, eosinophil count, and erythrocyte sedimentation rate increased, and the levels of seg neutrophils decreased. A biopsy revealed a leave-like parasite. The length was approximately 1.7–1.8-long, and the width was 0.5–0.8-long. The morphology of the posterior testis of the
F. hepatica was observed under a microscope. The fluke did not have hooks or spines but were characterized by suckers. The acetabulum wis located at the anterior part of the worm (
Fig. 2A). The granuloma contained acute and chronic inflammatory reactions accompanied by numerous eosinophilic invasive micronecrosis.
This patient came to our hospital with a liver abscess, but was diagnosed with abdominal muscular fascioliasis invoking RUQ pain. A parasitic infection caused by
Fasciola hepatica was confirmed by histological observation of biopsied material.
F. hepatica usually thrives in the hepatobiliary system, it often migrates aberrantly and causes ectopic fascioliasis [
12]. Ectopic fascioliasis in the various sites, such as the cecum, ascending colon, brain, eyes, spine, subcutaneous tissue, neck, and inguinal lymph nodes, have been reported in Korea [
13–
15]. In 2006, a very rare case of pancreatic infection was also reported in Korea [
16].
F. hepatica infection should be suspected in the differential diagnosis of parasitic infection based on the patient’s dietary history, eosinophilia, symptoms, and results of ELISA tests.
To molecularly identify
F. hepatica, PCR was performed on worm’s DNA sample. We also establish the phylogenetic classification of this specimen using the
F. hepatica 18S rRNA and
cox1 gene sequences. Their genetic mutations were compared with 17 previously reported strains. Amplification of the
cox1 gene was performed using primers
cox1 F 5′-TTTGCCTGG GTTTGGAGTTA-3′ and
cox1 R 5′-CCACACAACAGGATCCCATA-3′. The PCR conditions were optimized according to previous reports [
17]. Through PCR amplification, a target band with a length of approximately 273 bp was obtained for the
cox1 gene (
Fig. 2B). DNA sequencing was performed using Cosmo Genetech (Seoul, Korea). Nucleotide sequences were grouped according to host species (cattle, sheep, goat, and
Homo sapiens) and region (South Africa, Tunisia, Austria, Ecuador, France, and China). Isolates and reference sequences were used for multiple alignments and phylogenetic distances were calculated using MEGA version 6.0. Phylogenetic trees were constructed using the neighbor-joining method in the MEGA version 6.0 using
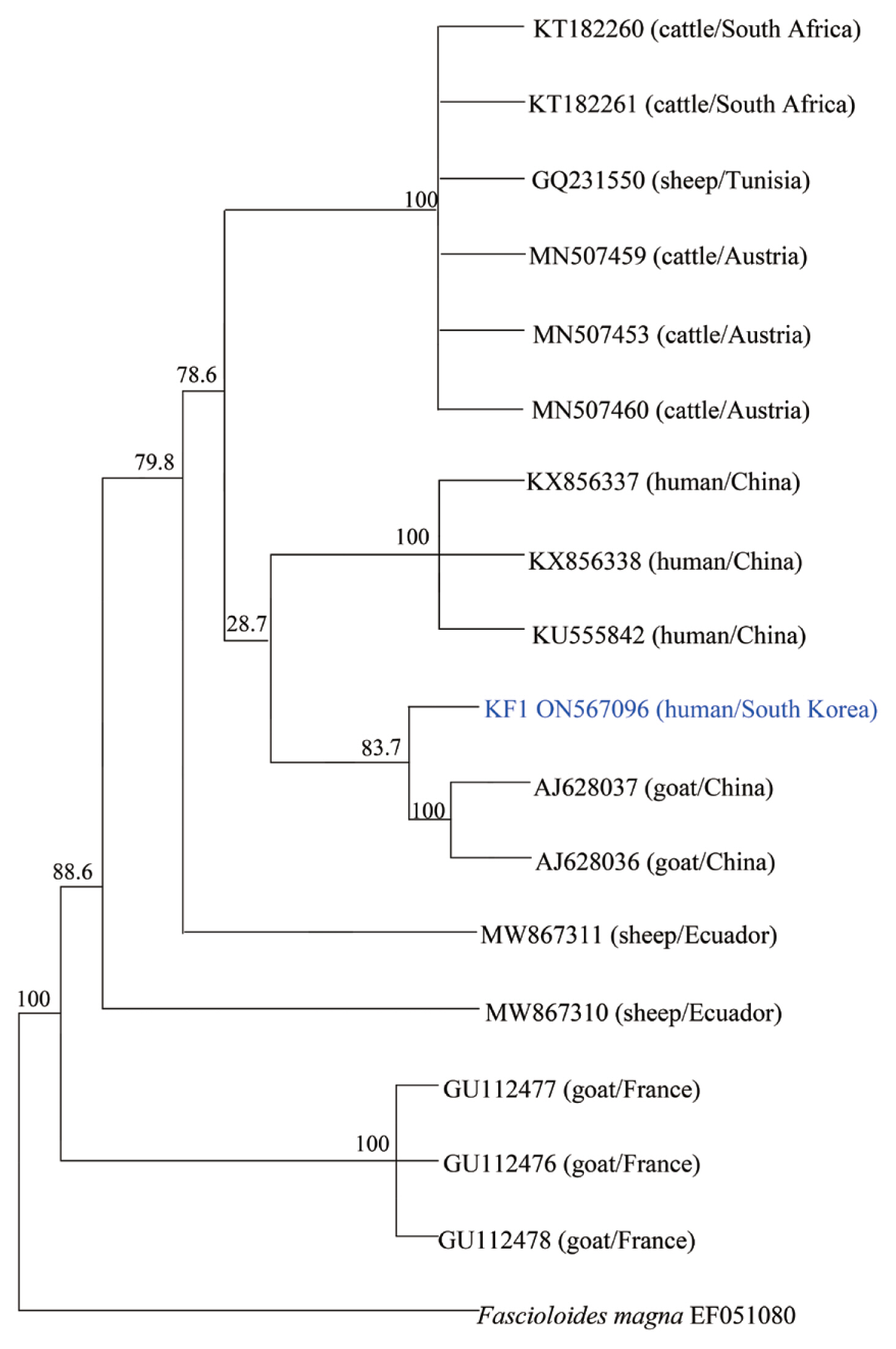
cox1 partial sequences isolated from a Korean patient (KF1) with 17 previously reported isolates. As shown in
Fig. 3, the phylogenetic tree clearly separated these homologs according to the host and region. KF1 was found to be closely related to
F. hepatica from goats in China, and the genetic characteristics of most of the isolates were geographically related, with no relationship was observed along with host differences.
Fascioliasis is generally diagnosed by detection of the characteristic eggs in feces. However, at low levels of infection, the sensitivity is very low even after repeated stool examinations. Serological tests are appropriate for diagnosing patients at the invasive stage, as they allow for early detection of infection. Anti-
Fasciola antibodies can be detected 2–4 weeks after infection [
6,
18].
A limitation related to the interpretation of the phylogenetic results in this study is that only one Korean isolate was used for the analysis. Phylogenetic analysis has revealed that goat cattle, and sheep share F. hepatica strain that can transmits to humans. In the context of the prevention of human fascioliasis, the pattern and phylogenetic aspects of parasitic infections appear to be very important. In Korea, there was genetic confirmation of F. hepatica, but there were no related data in the phylogenetic analysis. A study on the distribution of F. hepatica is needed for future epidemiological investigations of domestic infections.
Notes
-
The authors declare that they have no conflict of interest.
Fig. 1Abdominal computerized tomography (CT). (A) CT performed at the time of admission for evaluation of pain in the right upper quadrant (RUQ). (B) RUQ pain recurred after treatment of liver abscess. CT was taken to evaluate the abdominal wall mass. (C) Abdominal CT performed with new pain in the subcostal area. (D) There was no improvement after maintenance treatment with praziquantel. CT was taken again at Pusan National University Hospital. The arrows indicated a mass lesion.
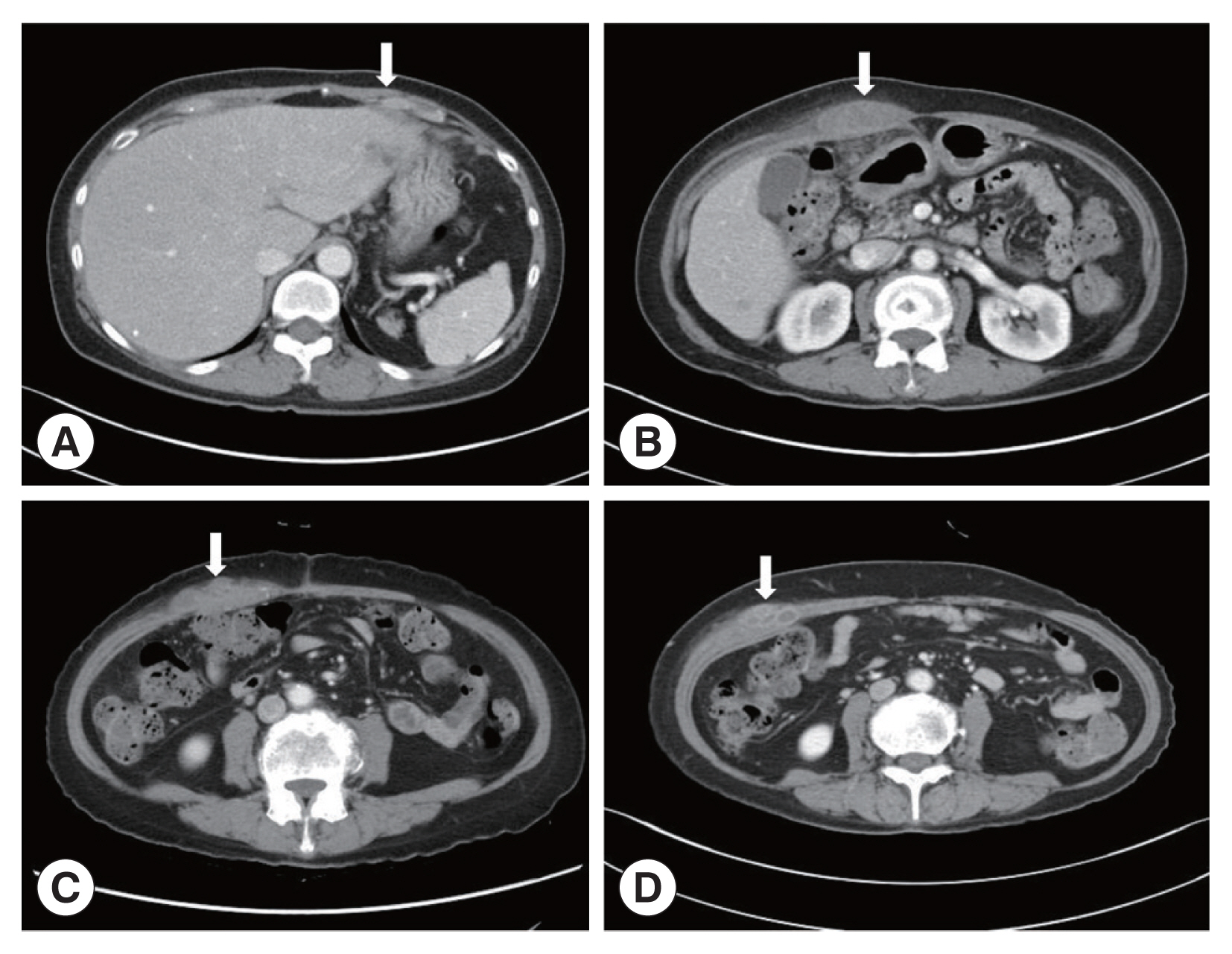
Fig. 2Morphological characteristics of liver fluke and electrophoretic analysis of PCR products. (A) Morphological characteristics of F. hepatica isolated from the patient. OS, oral sucker; A, acetabulum (ventral sucker); SV, seminal vesicle; PT, posterior testis. (B) Cox1 partial PCR product (M, 100-kb ladder).
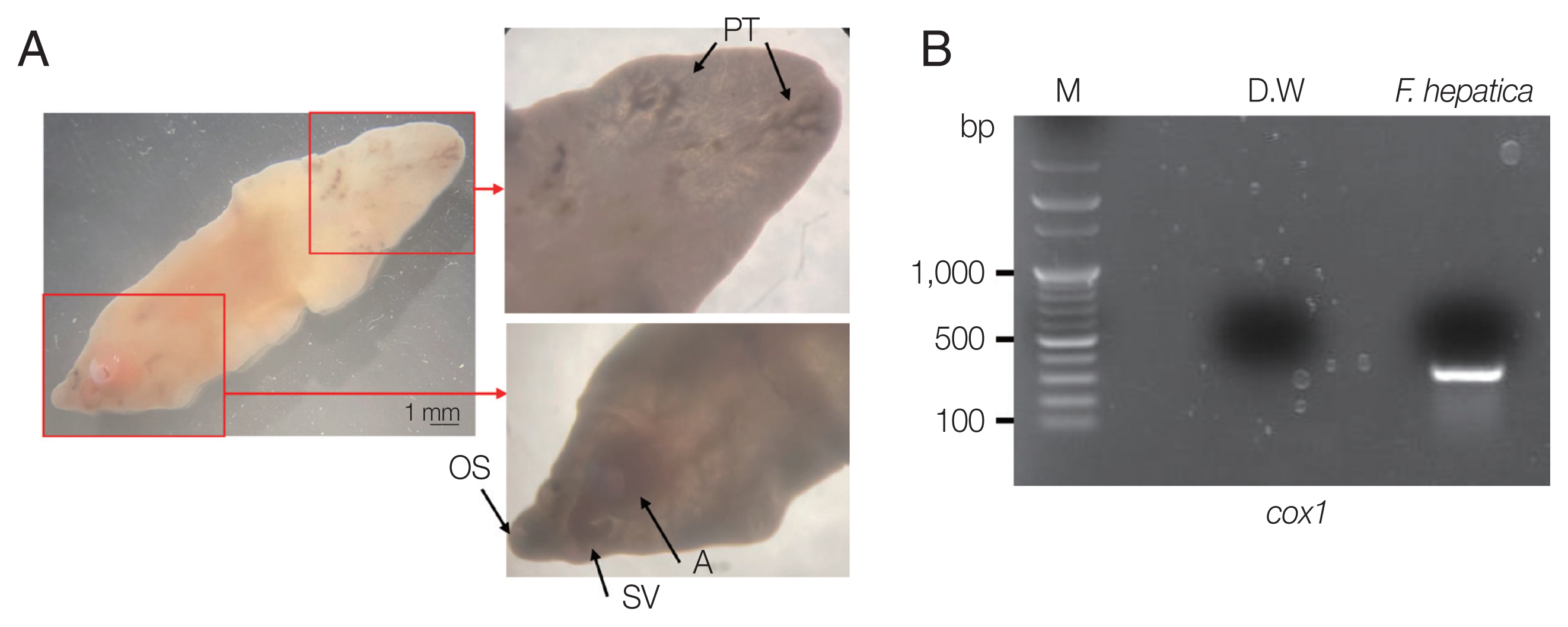
Fig. 3Phylogenetic tree of F. hepatica isolates generated using cox1 partial gene sequences. All of F. hepatica was represented by Genbank accession numbers. The genetic relationship between F. hepatica KF1 (ON567096) and 17 corresponding reference sequences was analyzed by the neighbor-joining method using MEGA, version 6.0.
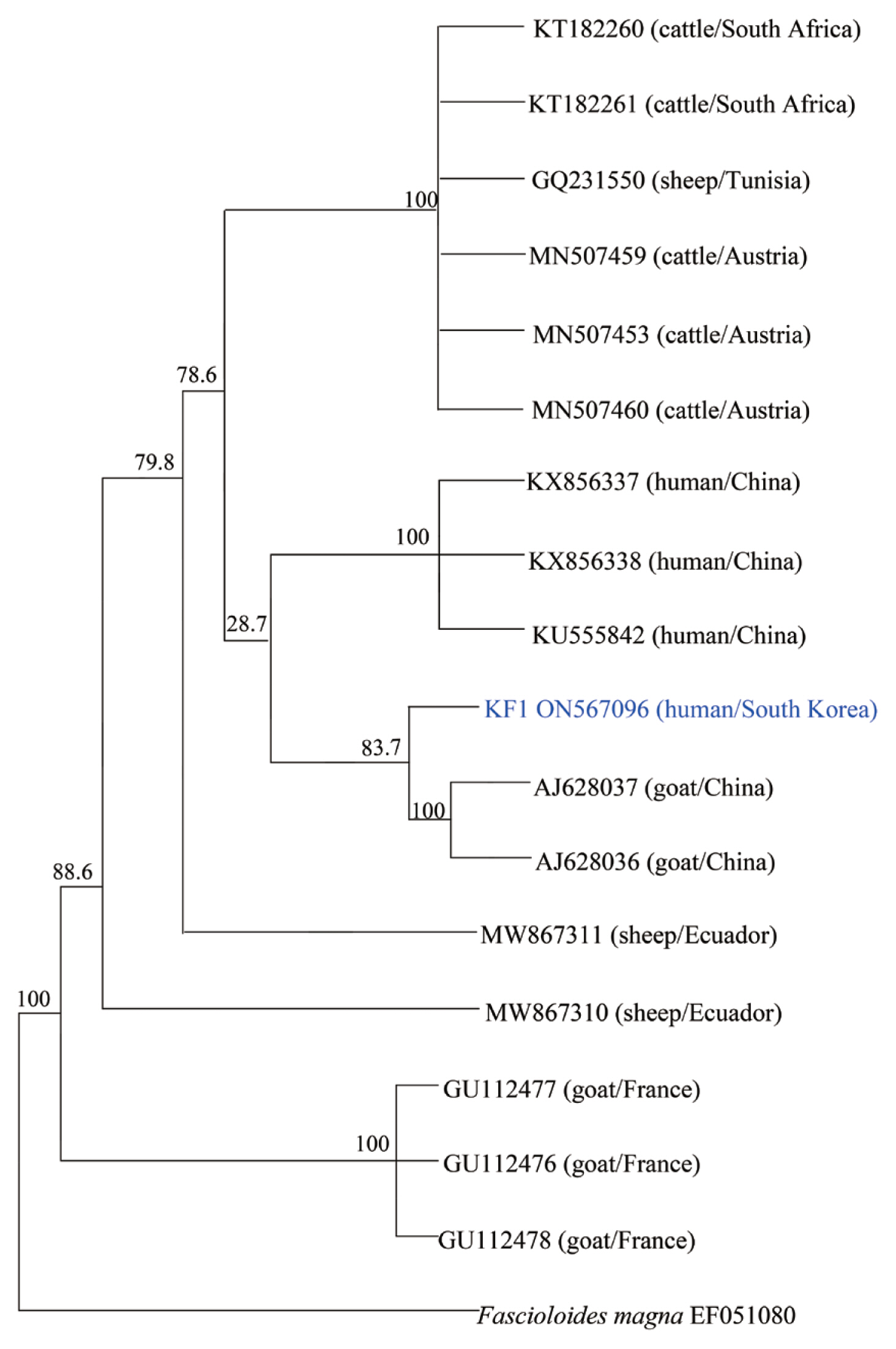
Table 1Information of human fascioliasis patients in Korea
Table 1
|
Year |
Sex |
Age |
Infection site |
First symptom |
Seconds symptom |
First/final diagnosis |
Gene study |
Reference |
|
1976 |
F |
42 |
Bile duct |
Urticaria, dyspepsia, epigastric discomfort |
Epigastric colicky pain attacks |
-/fascioliasis |
- |
[13] |
|
1984 |
F |
27 |
Cecum |
Nausea, vomiting, epigastric tenderness. |
Palpable mass |
Bowel obstruction/fascioliasis |
- |
[14] |
|
1991 |
F |
32 |
Left chest wall |
Palpable mass |
- |
-/fascioliasis |
- |
[19] |
|
1994 |
M |
28 |
Intraocular |
Headache, motor weakness |
Corneal edema with hyphemia |
Corneal edema/intraocular fascioliasis |
- |
[15] |
|
2006 |
F |
46 |
Pancreas |
Left upper quadrant pain |
Peripheral eosinophilia, hyperamylasemia, hyperlipasemia |
Acute pancreatitis/pancreatic fascioliasis |
- |
[16] |
|
2014 |
M |
87 |
Bile duct |
Abdominal pain and discomfort |
Peripheral eosinophilia |
Gallbladder stone and intraductal cholangiocarcinoma/fascioliasis |
ITS-1 |
[20] |
|
2015 |
F |
56 |
Colon |
Discomfort and pain in the abdomen |
Tenderness in the abdomen, eosinophilia |
Cysticercosis/fascioliasis |
- |
[12] |
|
2021 |
F |
66 |
Abdomen |
Right upper quadrant (RUQ) pain |
Pain in the subcostal region, eosinophilia |
Parametric infection/fascioliasis |
Cox-1 |
This study |
References
- 1. Ai L, Cai YC, Lu Y, Chen JX, Chen SH. Human cases of fascioliasis in Fujian Province, China. Korean J Parasitol 2017;55:55-60. https://doi.org/10.3347/kjp.2017.55.1.55
- 2. Caravedo MA, Cabada MM. Human fascioliasis: current epidemiological status and strategies for diagnosis, treatment, and control. Res Rep Trop Med 2020;11:149-158. https://doi.org/10.2147/RRTM.S237461
- 3. Parkinson M, O’Neill SM, Dalton JP. Endemic human fasciolosis in the Bolivian Altiplano. Epidemiol Infect 2007;135:669-674. https://doi.org/10.1017/S095026880600728X
- 4. Kaya M, Beştaş R, Cetin S. Clinical presentation and management of Fasciola hepatica infection: single-center experience. World J Gastroenterol 2011;17:4899-4904. https://doi.org/10.3748/wjg.v17.i44.4899
- 5. Aksoy DY, Kerimoglu U, Oto A, Erguven S, Arslan S, Unal S, Batman F, Bayraktar Y. Infection with Fasciola hepatica. Clin Microbiol Infect 2005;11:859-861. https://doi.org/10.1111/j.1469-0691.2005.01254.x
- 6. Mas-Coma S, Bargues MD, Valero MA. Diagnosis of human fascioliasis by stool and blood techniques: update for the present global scenario. Parasitology 2014;141:1918-1946. https://doi.org/10.1017/S0031182014000869
- 7. Schiappacasse RH, Mohammadi D, Christie AJ. Successful treatment of severe infection with Fasciola hepatica with praziquantel. J Infect Dis 1985;152:1339-1340. https://doi.org/10.1093/infdis/152.6.1339
- 8. Elliott T, Muller A, Brockwell Y, Murphy N, Grillo V, Toet HM, Anderson G, Sangster N, Spithill TW. Evidence for high genetic diversity of NAD1 and COX1 mitochondrial haplotypes among triclabendazole resistant and susceptible populations and field isolates of Fasciola hepatica (liver fluke) in Australia. Vet Parasitol 2014;200:90-96. https://doi.org/10.1016/j.vetpar.2013.11.019
- 9. Akhlaghi E, Mohammadi MA, Ziaali N, Baneshi MR, Nasibi S, Kamyabi H, Rostami S, Harandi MF. Morphometric and molecular study of Fasciola isolates from ruminants in Iran. Turkiye Parazitol Derg 2017;41:192-197. https://doi.org/10.5152/tpd.2017.5214
- 10. Agatsuma T, Arakawa Y, Iwagami M, Honzako Y, Cahyaningsih U, Kang SY, Hong SJ. Molecular evidence of natural hybridization between Fasciola hepatica and F. gigantica. Parasitol Int 2000;49:231-238. https://doi.org/10.1016/S1383-5769(00)00051-9
- 11. Park HJ, Choi GS, Jung M, Lee SU. Fasciola hepatica induced hepatic abscess treated with triclabendazole. Korean J Gastroenterol 2021;77:39-44. https://doi.org/10.4166/kjg.2020.152
- 12. Kim AJ, Choi CH, Choi SK, Shin YW, Park YK, Kim L, Choi SJ, Han JY, Kim JM, Chu YC, Park IS. Ectopic Human Fasciola hepatica infection by an adult worm in the mesocolon. Korean J Parasitol 2015;53:725-730. https://doi.org/10.3347/kjp.2015.53.6.725
- 13. Lee SH, Cho SY, Seo BS, Choe KJ, Chi JG. A human case of ectopic fascioliasis in Korea. Korean J Parasitol 1982;20:191-200. https://doi.org/10.3347/kjp.1982.20.2.191
- 14. Park CI, Kim H, Ro JY, Gutierrez Y. Human ectopic fascioliasis in the cecum. Am J Surg Pathol 1984;8:73-77. https://doi.org/10.1097/00000478-198401000-00008
- 15. Cho SY, Yang HN, Kong Y, Kim JC, Shin KW, Koo BS. Intraocular fascioliasis: a case report. Am J Trop Med Hyg 1994;50:349-353. https://doi.org/10.4269/ajtmh.1994.50.349
- 16. Lee OJ, Kim TH. Indirect evidence of ectopic pancreatic fascioliasis in a human. J Gastroenterol Hepatol 2006;21:1631-1633. https://doi.org/10.1111/j.1440-1746.2006.03185.x
- 17. Choi IW, Kim HY, Quan JH, Ryu JG, Sun R, Lee YH. Monitoring of Fasciola species contamination in water dropwort by cox1 mitochondrial and ITS-2 rDNA sequencing analysis. Korean J Parasitol 2015;53:641-645. https://doi.org/10.3347/kjp.2015.53.5.641
- 18. Gonzales Santana B, Dalton JP, Vasquez Camargo F, Parkinson M, Ndao M. The diagnosis of human fascioliasis by enzyme-linked immunosorbent assay (ELISA) using recombinant cathepsin L protease. PLoS Negl Trop Dis 2013;7:e2414. https://doi.org/10.1371/journal.pntd.0002414
- 19. Chang EC, Choi HL, Park YW, Kong Y, Cho SY. Subcutaneous fascioliasis: a case report. Korean J Parasitol 1991;29:403-405. https://doi.org/10.3347/kjp.1991.29.4.403
- 20. Kang BK, Jung BK, Lee YS, Hwang IK, Lim H, Cho J, Hwang JH, Chai JY. A case of Fasciola hepatica infection mimicking cholangiocarcinoma and ITS-1 sequencing of the worm. Korean J Parasitol 2014;52:193-196. https://doi.org/10.3347/kjp.2014.52.2.193
Citations
Citations to this article as recorded by

- Toxocara canis and Fasciola hepatica Co-Infection Leading to Hepatic Abscess: A Case Report
Seung Wan Kim, Byoung Kuk Jang
Journal of Korean Medical Science.2023;[Epub] CrossRef