Abstract
Clonorchis sinensis is commonly found in East Asian countries. Clonorchiasis is prevalent in these countries and can lead to various clinical symptoms. In this study, we used overlap extension polymerase chain reaction (PCR) and the Xenopus laevis oocyte expression system to isolate a cDNA encoding the choline transporter of C. sinensis (CsChT). We subsequently characterized recombinant CsChT. Expression of CsChT in X. laevis oocytes enabled efficient transport of radiolabeled choline, with no detectable uptake of arginine, α-ketoglutarate, p-aminohippurate, taurocholate, and estrone sulfate. Influx and efflux experiments showed that CsChT-mediated choline uptake was time- and sodium-dependent, with no exchange properties. Concentration-dependent analyses of revealed saturable kinetics consistent with the Michaelis–Menten equation, while nonlinear regression analyses revealed a Km value of 8.3 μM and a Vmax of 61.0 pmol/oocyte/h. These findings contribute to widen our understanding of CsChT transport properties and the cascade of choline metabolisms within C. sinensis.
-
Key words: Clonorchis sinensis, choline, choline transporter, solute carrier family 5 member 7
Introduction
Clonorchiasis is a food-borne trematode disease caused by the liver fluke,
Clonorchis sinensis. Liver fluke infections are highly prevalent in East Asian countries, including Korea [
1], China [
2], several Southeast Asian countries [
3], and East Russia [
4]. The number of estimated
C. sinensis infections in these countries approximated 15 million cases, primarily affecting individuals who consume uncooked freshwater fish. Additionally, more than 200 million people are at risk of infection [
4]. Despite its high prevalence, clonorchiasis is often unnoticed because it is asymptomatic or presents with mild symptoms, without specific signs at the early stages of infection [
5]. Nevertheless, clonorchiasis affects the liver and bile duct, with various clinical consequences such as bile duct hyperplasia, obstructive jaundice, liver enlargement, cirrhosis, and the formation of calculi. In severe cases, chronic infection can lead to cholecystitis, cholangitis, and even cholangiocarcinoma [
6].
The primary drug of choice for treating
C. sinensis infections is praziquantel (PZQ), which targets the melastatin ion channel on the parasite’s syncitial membrane (confirmed in schistosomes), leading to the activation of transient calcium entry and resulting in worm paralysis [
7]. The external surface of trematodes plays various essential biological roles, including nutrient absorption, protection, and sensory input, all of which are essential for the parasite’s survival within the host. Thus, understanding the biological features of trematodes is critical for the prevention and treatment of related diseases. Among these features, potential candidate is the transporter system involved in nutrient absorption, which remains largely unknown in trematodes.
An alternative method of killing parasitic trematodes involves targeting their neuromuscular system, which is specifically targeted by major anthelmintics such as pyrantel, monepantel, and levamisole [
8]. These drugs affect the maturation and locomotion of the parasite, leading to hindered survival within the host. These anthelmintics act as agonists to the nicotinic acetylcholine receptor (nAchR) on the muscle cells of the parasites [
9], which interrupts the cholinergic system, resulting in the inhibition of the trematode’s neuromuscular activity [
8]. In trematodes, acetylcholine (Ach) acts as a major neurotransmitter, and its synthesis pathway likely begins with the uptake of choline from the host. Similar to the abovementioned anthelmintics, tribendimidine has demonstrated its efficacy against
C. sinensis infection by acting as an L-subtype nAchR agonist [
10]. Therefore, tribendimidine is a viable treatment option for
C. sinensis and other helminth infections [
4,
11]. In
Schistosoma mansoni, choline plays a significant role in bridging the metabolism of glycine and serine to facilitate both folate biosynthesis and lipid metabolism through the Kennedy pathway [
12,
13]. Additionally, choline is involved in the methylation pathway [
14], and it serves as a by-product in the production of Ach for neurotransmission [
9].
Overall, choline is considered an essential nutrient that plays vital roles in maintaining cell membrane stability, facilitating cell signaling, and supporting neurotransmitter synthesis [
15]. The transport of choline across cell membranes is mediated by various protein families, including the high-affinity choline transporter [
16]. Although choline transporters have been extensively studied in other parasites, research on the choline transporter in
C. sinensis remains limited [
17–
19]. Developing new drugs against this parasite will require a better understanding of the characteristics of this transporter. Recent research in a wide range of diseases has shown a growing interest in solute carrier and ATP-binding cassette transporters as potential drug targets [
20]. The objectives of this study was to clone and characterize the choline transporter in
C. sinensis; and to explore its functional properties using
Xenopus laevis oocytes.
Materials and Methods
Computational analysis
The nucloetide sequence (accession number DF144738.1) of the putative high-affinity choline transporter of
C. sinensis (CsChT) was acquired through a search on the National Center for Biotechnology Information (NCBI). The corresponding amino acid sequence encodes the solute carrier family 5 member 7 (high-affinity choline transporter, SLC5A7; accession number: GAA57421.1). Other high-affinity choline transporter genes from flukes were obtained from the NCBI database, and the sequences were aligned using the Clustal W program (
http://clustalw.ddbj.nig.ac.jp). The evolutionary history of the choline transporter in flukes was inferred using MEGA11 software (
http://www.megasoftware.net) by implementing maximum-likelihood analysis with a bootstrap test of 1,000 pseudo replications to enhance robustness.
The topology of membrane spanning was determined using the TMpred server (
https://ftp.vital-it.ch/pub/software/unix/tmpred/www/TMPRED_form.html), which predicted the presence of 13 potential transmembrane domains. The tertiary structure of CsChT was further analyzed by submitting the putative CsChT sequences to the SWISS-MODEL server (
https://swissmodel.expasy.org), a homology-base modeling tool that identified suitable structure templates as described elsewhere [
21]. We then implemented Ramachandran plots and ERRAT to assess the quality of the tertiary structure model and identify potential errors. The generated model was refined to improve accuracy using a combination of 2 programs, Mode Refiner and Galaxy Refine. Finally, the final predicted structure was selected and visualized using UCSF CHIMERA software.
To obtain adult
C. sinensis worms, we collected specimens of
Pseudorasbora parva and
Gnathopogon coreanus from the Jinju Nam River, an area in Korea that is highly associated with metacercariae. The fishes were digested enzymatically using an artificial digestive solution consisting of 0.6% pepsin and 0.7% HCl at pH 2.0. The digestion process was carried out for 6 h in a shaking water bath at 37°C with gentle agitation. Undigested material were removed by filtration using a five-fold gauze. The residue was washed 5 times with a normal saline solution (0.85% NaCl) to eliminate the digestive solution. Metacercariae were then isolated by filtering the mixture through a 200-μm stainless wire mesh, resulting in the collection of approximately 500 metacercariae. Experimental infections were carried out using 5–6-week-old male FVB/NJ mice (Central Lab Animal Inc., Seoul, Korea), which were orally infected with 30 metacercariae. Six weeks after infection, the mice feces were examined for the presence of eggs using the formalin-ether sedimentation method [
22]. Finally, adult
C. sinensis worms were collected from the liver margin and bile ducts approximately 8 weeks after the initial infection. The animal experiment protocol was reviewed and approved by the institutional animal care and use committee of Inha University (approval no. INHA161208-460), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The mice were cared for at the Inha University Animal Facility following the National Animal Care Policies (Accredited Unit, Korea FDA; unit number 36).
A total of 500 ng of RNA extracted from adult worm was used to synthesize cDNA via reverse transcription. The process used 5 units of Avian Myeloblastosis Virus reverse transcriptase XL kit (Takara, Kusatsu, Japan), 10 mM of dNTP, and 2.5 μM of oligo dT primers in a total volume of 30 μl. The mixture was then incubated at 42°C for 30 min, followed by a temperature increase to 99°C for 5 min. The mixture was then held at 4°C.
The full length of the putative CsChT gene was generated using overlap extension PCR, which involved the designing of 3 sets of primers to generate 3 gene segments, each with overlapping regions of at least 20 base pairs. The reaction mixture for each segment consisted of 3 μl of cDNA, 3 μl of dNTP (2.5 mM each), 1 unit of Ex Taq DNA polymerase (Takara), 3 μl of 10×PCR reaction buffer, 1 μl of forward and reverse primer (10 μM) each (
Supplementary Table S1), and DNase/RNase-free water to adjust the final volume to 30 μl. The thermocycler conditions were as follows: predenaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 45 sec, and a final extension at 72°C for 5 min. The amplicon was purified using a Gel Extraction Kit (GeneAll Biotechnology, Seoul, Korea) and subcloned into a pGEM-T easy TA cloning vector (Promega, Madison, WI, USA) for sequence confirmation. After verifying the sequence (Macrogen, Seoul, Korea), the plasmid DNA was digested with
EcoRI to obtain the overlapping PCR templates (
Supplementary Fig. S1A). The reaction mixture for overlapping PCR consisted of 1 μl of each PCR segment, 3 μl of dNTPs (2.5 mM each), 2 units of LA taq DNA polymerase (Takara), 3 μl of 10×PCR reaction buffer, 2 μl of 25 mM MgCl
2, 1 μl of first forward and third reverse primers (10 μM) each, and DNase/RNase-free water to adjust the final volume to 30 μl. The overlapping PCR conditions were as follows: predenaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec, extension at 72°C for 2 min, and a final extension at 72°C for 5 min (
Supplementary Fig. S1B).
To measure choline uptake properties, we employed the
Xenopus laevis oocyte expression system, which has been widely used to characterize transporters, as described previously [
23]. Capped cRNA was synthesized in vitro using the mMESSAGE mMACHINE T7 Transcription Kit (ThermoFisher Scientific, Carisbad, MA, USA) using cDNA linearized with the
Xho I (Enzynomics, Daejeon, Korea) restriction enzyme. Fifty nanoliters of cRNA was injected into defolliculated oocytes at a rate of 50 ng per oocyte, while the control group was injected with an equal volume of distilled water. The injected oocytes were then incubated at 18°C in Barth’s solution (88 mM NaCl, 1 mM KCl, 0.33 mM Ca(NO
3)
2, 0.4 mM CaCl
2, 0.8 mM MgSO
4, 2.4 mM NaHCO
3, 10 mM HEPES, pH 7.4) supplemented with 50 μg/ml gentamicin and 2 mM of sodium pyruvate. After an incubation period of 2 to 3 days, CsChT expression was assessed by measuring the uptake of [
3H] choline (PerkinElmer, Waltham, MA, USA) at 22–25°C in ND96 solution (96 mM NaCl, 2 mM KCl, 2 mM CaCl
2, 1 mM MgCl
2, 5 mM HEPES, pH 7.4) for 1 h. The uptake reaction was stopped by the addition of ice-cold ND96 solution, and the oocytes were subsequently washed 4 times with the same solution to remove residual substrate. The oocytes were dissolved with 10% SDS solution under vigorous shaking for 1 h, followed by the addition of 2 ml of the scintillation cocktail, Ultima Gold AB (PerkinElmer). The radioactivity of dissolved oocytes was measured using a MicroBeta
2 β-Counter (PerkinElmer).
The
trans-stimulatory effect of CsChT on the efflux of radiolabeled choline was investigated by preloading CsChT-expressing oocytes with [
3H] choline (10 μM in the medium) for 90 min. The oocytes were then transferred to medium that was either contained excess amount of (100 μM and 1,000 μM each) or lacked unlabeled choline and incubated for 1 h [
24].
The kinetic parameters associated with the CsChT-mediated uptake of choline were estimated using the following equation: v=Vmax×S/(Km+S), where v represents the substrate uptake rate (pmol/h/oocyte), S denotes the substrate concentration in the medium (μM), Km represents the Michaelis–Menten constant (μM), and Vmax represents the maximum uptake rate (pmol/h/oocyte). To determine these kinetic parameters, the equation was fitted to the choline transport velocity, which was obtained by subtracting the transport velocity of choline in noninjected oocytes from that in CsChT-expressing oocytes. This fitting graph was constructed using an iterative nonlinear least squares method using the MULTI program [
25]. The input data, consisting of weighted values using the reciprocal of the observed values, and then fitted using the Damping Gauss Newton method [
25]. Subsequently, the fitted line was transformed into the 1/S versus 1/V format for Lineweaver–Burk analysis.
Results
Schematic characteristics and tertiary structure of CsChT
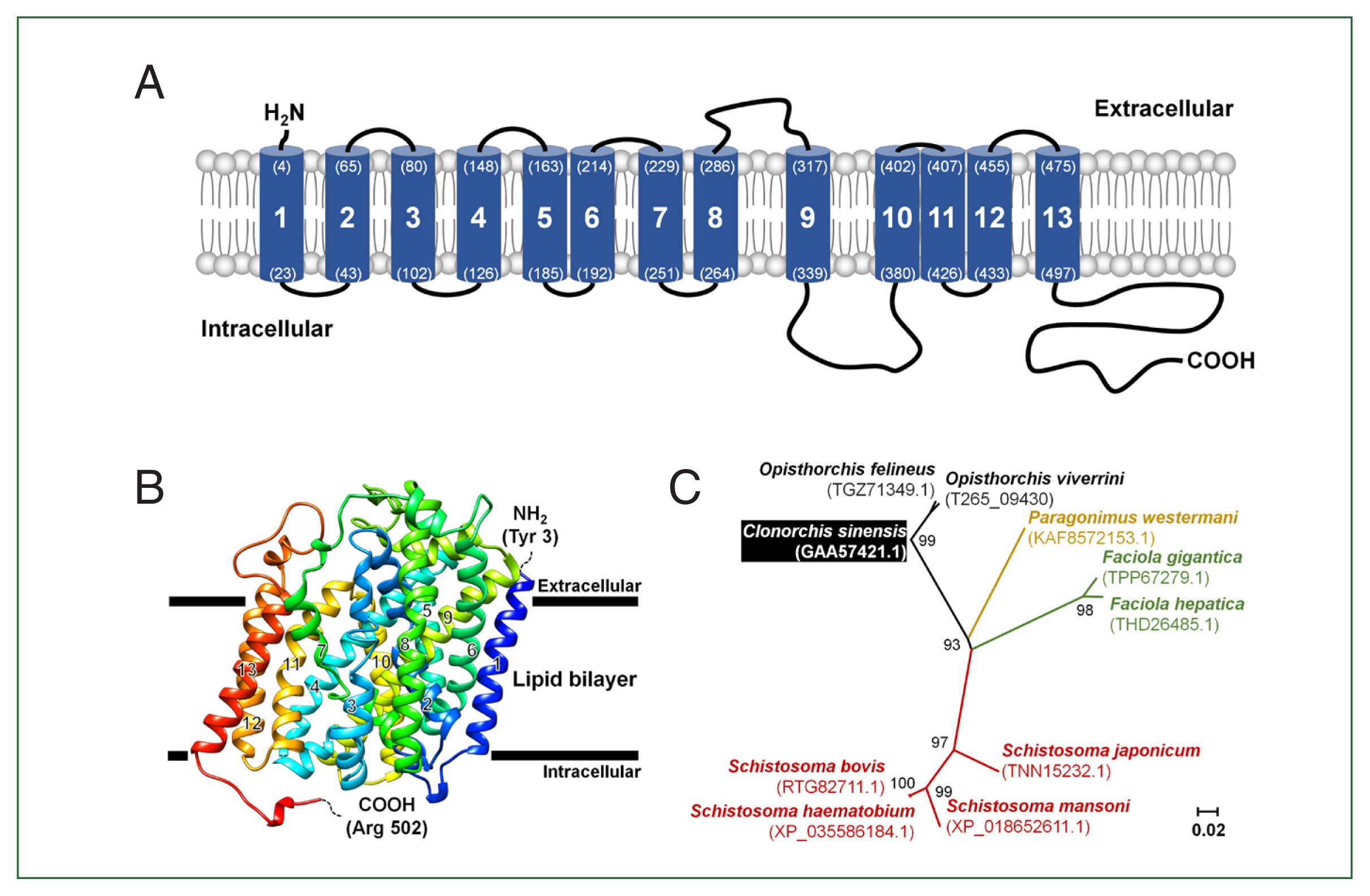
The full length of CsChT (GAA57421) is composed of 1,710 base pairs, which encodes 569 amino acid residues (aa.) within an open reading frame that encodes a protein weighing 62.7 kDa. One to three amino acids of the N-terminus tail is positioned in the extracellular region and the region is predicted to contain 13 putative transmembrane segments. The C-terminus tail spans amino acid residues 498–569 and is located within the intracellular environment (
Fig. 1A;
Supplementary Fig. S2).
To determine the tertiary structure of CsChT using the SWISS-MODEL server, we found the human sodium/glucose cotransporter 2 (hSGLT2, PDB ID: 7vsi.1.A) to be an excellent template for structural fitting. Analysis through a Ramachandran plot indicated that 90.1% and 0.2% of the model occupied favorable and disallowed regions, respectively. The initial ab initio model structure had a quality verification score of 87.6%, according to ERRAT results. Model refinement significantly improved the 3-dimensional structure, resulting in 95.4% and 0.2% of the model occupying favorable and disallowed regions, respectively, based on the Ramachandran plot. ERRAT analysis of the refined model indicated a final allowed CsChT structure quality of 90.3% (
Fig. 1B). The final tertiary structure, consisting of amino acid residues 3 to 502 and encompassing 13 α-helical transmembrane domains, is shown in
Fig. 1B.
The relationships between putative choline transporter-related genes were determined by comparing the CsChT sequence to those of high-affinity-related genes from other flukes, which were obtained from the NCBI database. Sequence analysis indicates the following levels of homology among choline transporter-related proteins of other flukes to CsChT:
Fasciola gigantica (TPP67279.1), 71%;
Fasciola hepatica (THD26485.1), 72%;
Opisthorchis felineus (TGZ71349.1), 97%;
Paragonimus westermani (KAF8572153.1), 76%;
Schistosoma bovis (RTG82711.1), 70%;
Schistosoma haematobium (XP_035586184.1), 69%;
Schistosoma japonicum (TNN15232.1), 72%; and
Schistosoma mansoni (XP_018652611.1), 63% (
Fig. 1C;
Table 1).
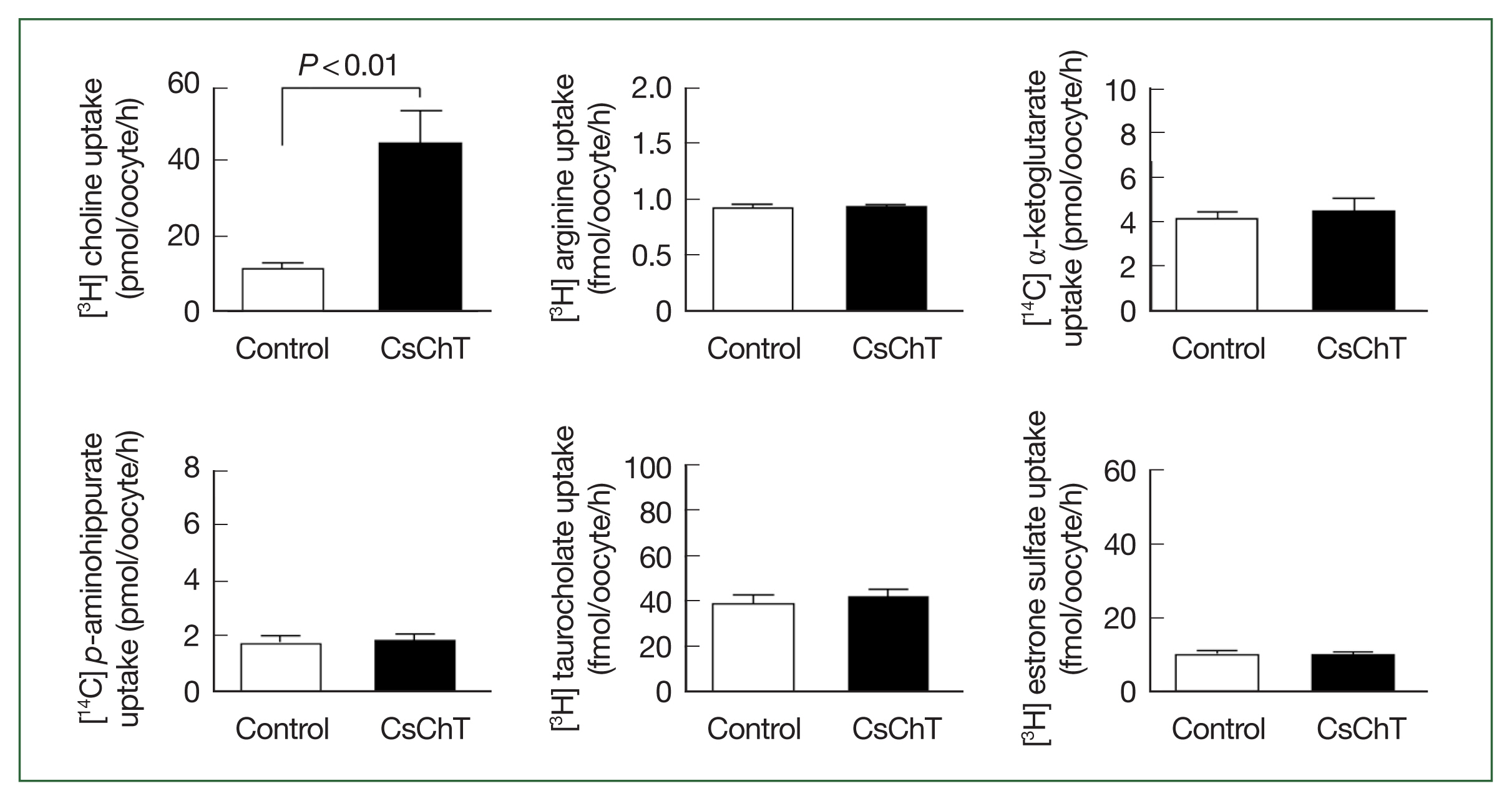
The transport properties of various substrates via CsChT were investigated using the
Xenopus oocyte expression system. Specifically, we examined the uptake rates of isotope-labeled choline (for choline transporter), arginine (an amino acid), α-ketoglutarate (a dicarboxylate), p-aminohippurate (an organic anion), taurocholate (a bile acid), and estrone sulfate (a steroid hormone). The results reveal that the uptake rate of [
3H] choline in oocytes expressing CsChT was approximately 4.5 times higher than that of the control group (control: 9.6± 1.2 pmol/oocyte/h, CsChT: 43.6±3.6 pmol/oocyte/h). In contrast, we observed no significant uptake of amino acids, organic anions, dicarboxylates, steroid hormones, and bile acids (
Fig. 2).
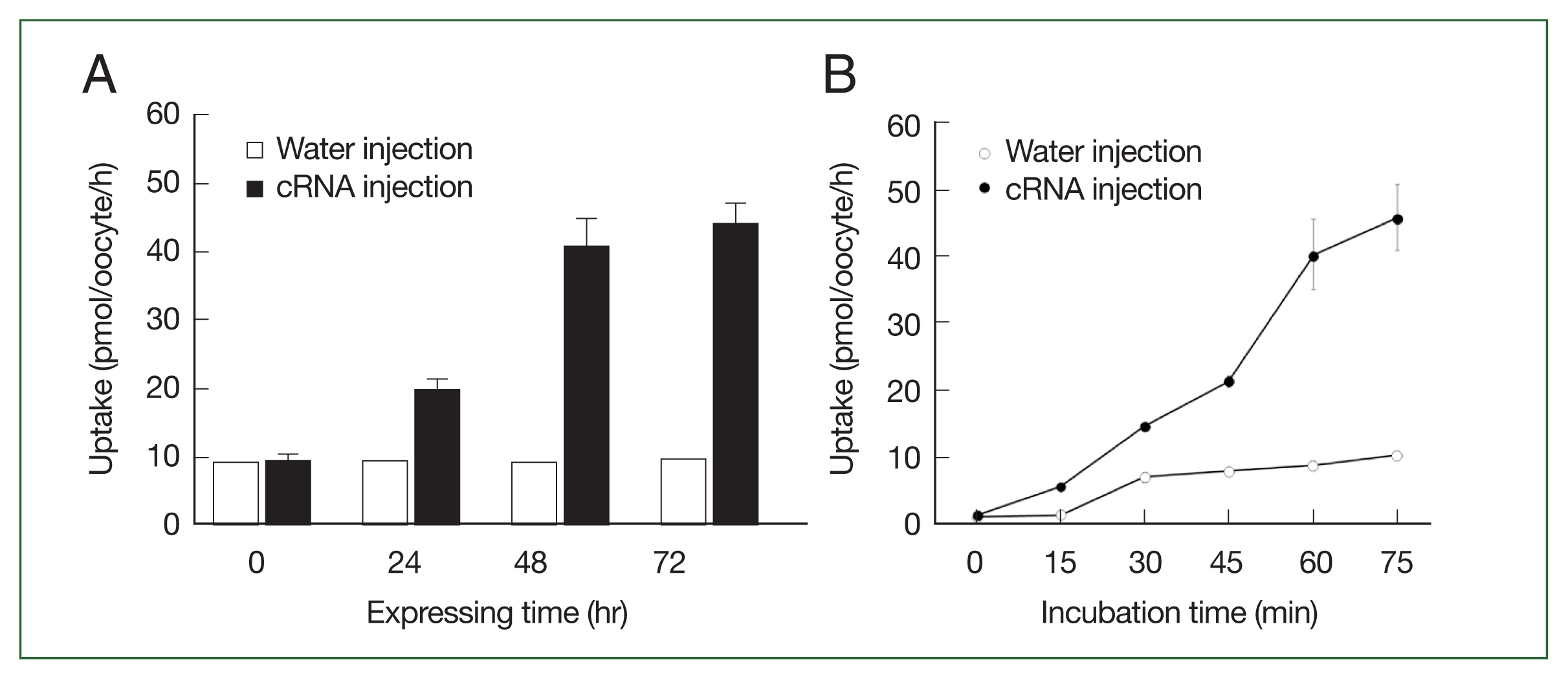
The CsChT-mediated uptake of [
3H] choline was dependent on both expression time (i.e., increasing for 1–3 days,
Fig. 3A) and incubation time (i.e., uptake intensified between 15 to 75 min,
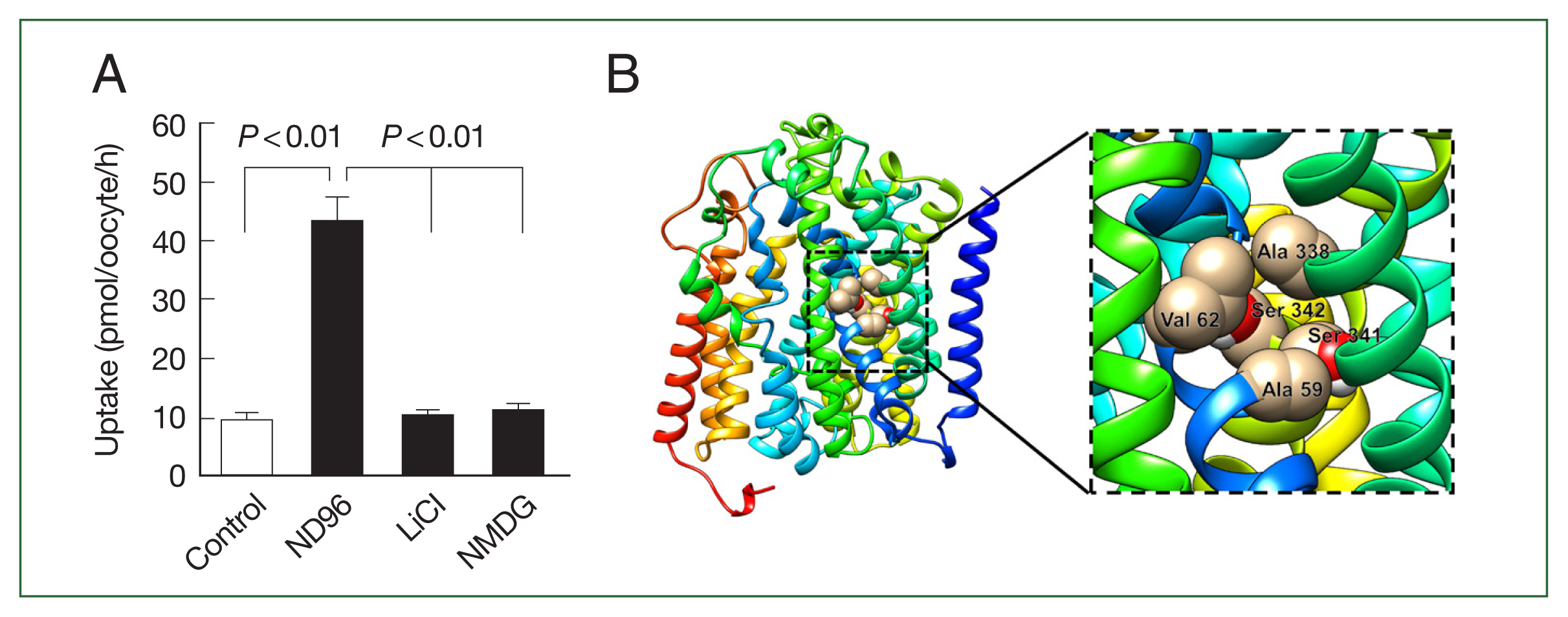
Fig. 3B). Similarly, choline uptake was dependent on sodium ions, which we confirmed by replacing the extracellular sodium content (ND96, containing 96 mM Na
+ ion) with equimolar amounts of LiCl and NMDG (N-methyl-D-glucosamine). Notably, CsChT-mediated choline uptake was significantly diminished during one hour in the presence of LiCl and NMDG (
Fig. 4A). We searched for putative sodium ion binding residues within the conserved domain database of NCBI, and we found a typical solute-binding domain within CsChT that is homologous to that of Na
+- and Cl
−-dependent choline cotransporters. We identified several sodium ion binding residues (Ala 59, Val 62, Ala 338, Ser 341, and Ser 342) within the conserved domain of CsChT, demonstrating that these residues are highly conserved across diverse organisms (
Fig. 4B). These residues are located within the second and ninth transmembrane cores, with 2 serine residues positioned in the intracellular region (
Fig. 1B). The spatial arrangement of these sodium-binding residues creates a pocket that is conducive to sodium ion binding, which is essential for transporting choline (
Fig. 4B).
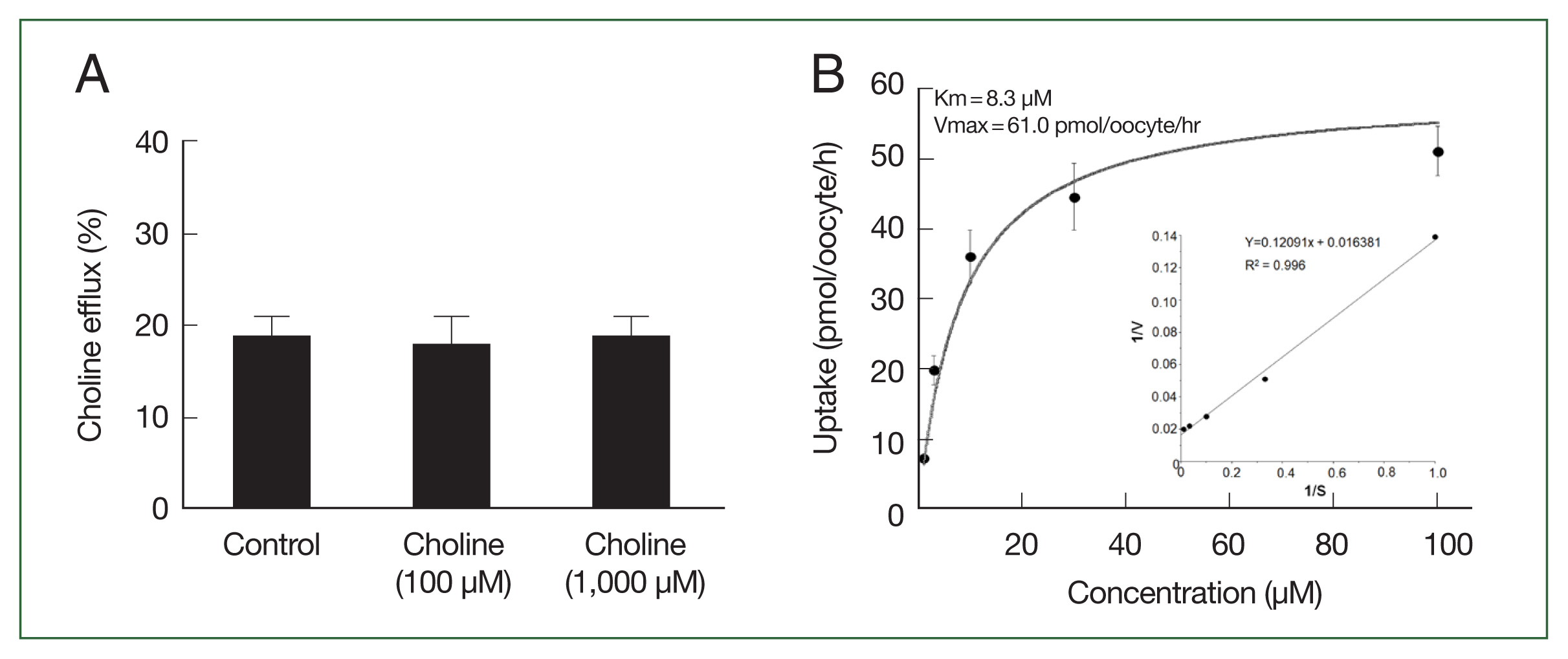
We examined the transstimulatory property of choline transport via CsChT by adding choline to the medium at concentrations of 100 μM and 1,000 μM, which are 10 and 100 times higher than preloaded choline concentration, respectively. We did not observe any significant CsChT-mediated efflux of preloaded isotope-labeled choline (
Fig. 5A). The uptake of [
3H] choline via CsChT was concentration-dependent, displaying saturable kinetics that followed the Michaelis–Menten equation (
Fig. 5B). Lineweaver–Burk analysis of choline uptake yielded a Km value of 8.3 μM and a Vmax value of 61.0 pmol/oocyte/h (
Fig. 5B).
Discussion
Choline is an essential nutrient that is involved in various biological processes in different organisms. Specifically, it plays critical roles in membrane synthesis through lipid metabolism [
16], neurotransmission [
26], and in signal transduction via lecithin [
27]. The choline transporter is one of 12 members of the solute carrier family 5 (SLC5), which are found in various organisms. These transporters are essential for transporting various substrates, including sugars, vitamins, amino acids, and smaller organic ions such as choline. Here, we investigated the functional role of the
C. sinensis choline transporter (CsChT; SLC5A7). Our findings reveal that CsChT actively transports choline in a sodium- and time-dependent manner and with a Km value of 8.3 μM. The corresponding Km values of high-affinity choline transporters in
Schistosoma mansoni,
Trypanosoma brucei,
Plasmodium falciparum, and
Hymenolepis diminuta are 36 μM, 2.0 μM, 25.0 μM, and 500 μM, respectively [
19,
28,
29]. In 2 parasitic helminths (
Schistosoma mansoni and
Echinostoma caproni), choline is taken up from the host and is utilized for lipid biosynthesis [
18,
30]. Similarly, choline and choline-derivates, such as betaine, phosphocholine (PC), and glycerophosphocholine (GPC) are involved in lipid metabolism of various tissues in
Faciola hepatica infecting rats [
31]. Hence, similar to other trematodes, choline is probably utilized by
C. sinensis for lipid biosynthesis via the lipid metabolism pathway.
Another essential role of choline is that of a neurotransmitter. The high-affinity choline transporter is a member of the sodium ion/glucose cotransporter family (SLC5) and is present on the presynaptic terminal of cholinergic neurons, where it transports choline, which is the precursor of acetylcholine [
32]. The presynaptic terminal contains a network of ion channels and proteins that provide the machinery for vesicular release and voluntary muscle activation. Once acetylcholine acts as a neurotransmitter, it is converted by acetylcholinesterase back into choline for reuse [
33]. Therefore, choline transport is essential for maintaining biostability, making it a promising target for antihelminthic drugs [
34]. CsChT exhibits molecular properties of a symporter, that is, the uptake of choline is sodium ion-dependent. It is likely that the binding of the sodium ion to conserved residues in CsChT (similar those in other organisms’ choline transporters) serves as the rate-limiting step in the synthesis of acetylcholine, which contributes to the maintenance of biostability [
32].
Praziquantel, the primary drug used to treat parasitic infections caused by trematodes or cestodes [
35], is taken by approximately 100 million people worldwide every year [
36]. While PZQ is generally considered safe, its common side effects include abdominal pain, diarrhea, sleep disorder, nausea, vomiting, and headaches, as well as less common side effects such as severe hypersensitive reactions that require specific medical treatment, including skin rash, dyspnea, hypotensive shock, and anaphylactic reactions [
36]. To minimize the risk of hypersensitive reactions, alternative drugs like albendazole and oxaminiquine are sometimes used for the treatment of schistosomiasis, one of the diseases caused by trematodes [
37]. Similarly, alternative drugs such as tribendimidine, albendazole, and mebendazole have been substituted for PZQ in cases of clonorchiasis with other coinfecting helminths. However, the chlonorchiasis treatment success rate of tribendimidine is only 33%, compared to >90% for that of PZQ [
38]. Similarly, albendazole has demonstrated limited effectiveness, while mebendazole is poorly absorbed [
4,
39]. Therefore, effective alternative drugs for clonorchiasis is scarce, with PZQ causing side effects and hypersensitive reactions. Hence, there is an urgent need to develop new drugs that can broaden treatment options and minimize adverse reactions of clonorchiasis patients.
In this study, for the first time, we demonstrate evidence of a choline transporter in
C. sinensis. Our results also provide information regarding the initiation of choline metabolism in
C. sinensis. The characteristics of the
C. sinensis choline transporter described in this study, suggest that it will be possible to develop new drugs for clonorchiasis [
40]. Moreover, our findings suggest that the choline transporter can be used to screen for potential anthelmintics that target cell signaling, particularly inhibitors that block choline uptake [
40].
Notes
-
Author contributions
Conceptualization: Won JY, Cha SH
Data curation: Won JY, Cha SH
Formal analysis: Won JY, Louis JM, Roh ES, Cha SH, Han JH
Funding acquisition: Cha SH
Methodology: Cha SH
Resources: Cha SH, Han JH
Supervision: Cha SH, Han JH
Validation: Won JY
Writing – original draft: Han JH
Writing – review & editing: Louis JM, Cha SH, Han JH
-
The authors declare no competing interests.
Supplementary Information
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF-2017R1D1A1B03034588). Adult Xemopus laevies was obtained from the Korean Xenopus Resource Center for Research.
Fig. 1Schematic representation of the CsChT characteristics. (A) The predicted topology of the full-length CsChT (1–569 aa.) showed 13 transmembrane segments with the short N-terminus (1–3 aa.) facing the extracellular region and the large C-terminus (498–569 aa.) facing the intracellular region. The number of transmembrane domains and amino acid positions are shown in parenthesis with the residue number. (B) CsChT homology-based model on the human sodium/glucose cotransporter 2 (hSGLT2, PDB ID: 7vsi.1.A) structure. The homology base model was constructed from Tyr 3 (N-terminus, blue color) to Arg 502 (C-terminus, red color). The horizontal bars represent the expected lipid bilayer boundaries of the 13 transmembrane domains. (C) Phylogenetic tree of putative choline transporters in flukes (NCBI accession number shown in parenthesis) constructed using MEGA11 by ML method shows the distance of relationship.
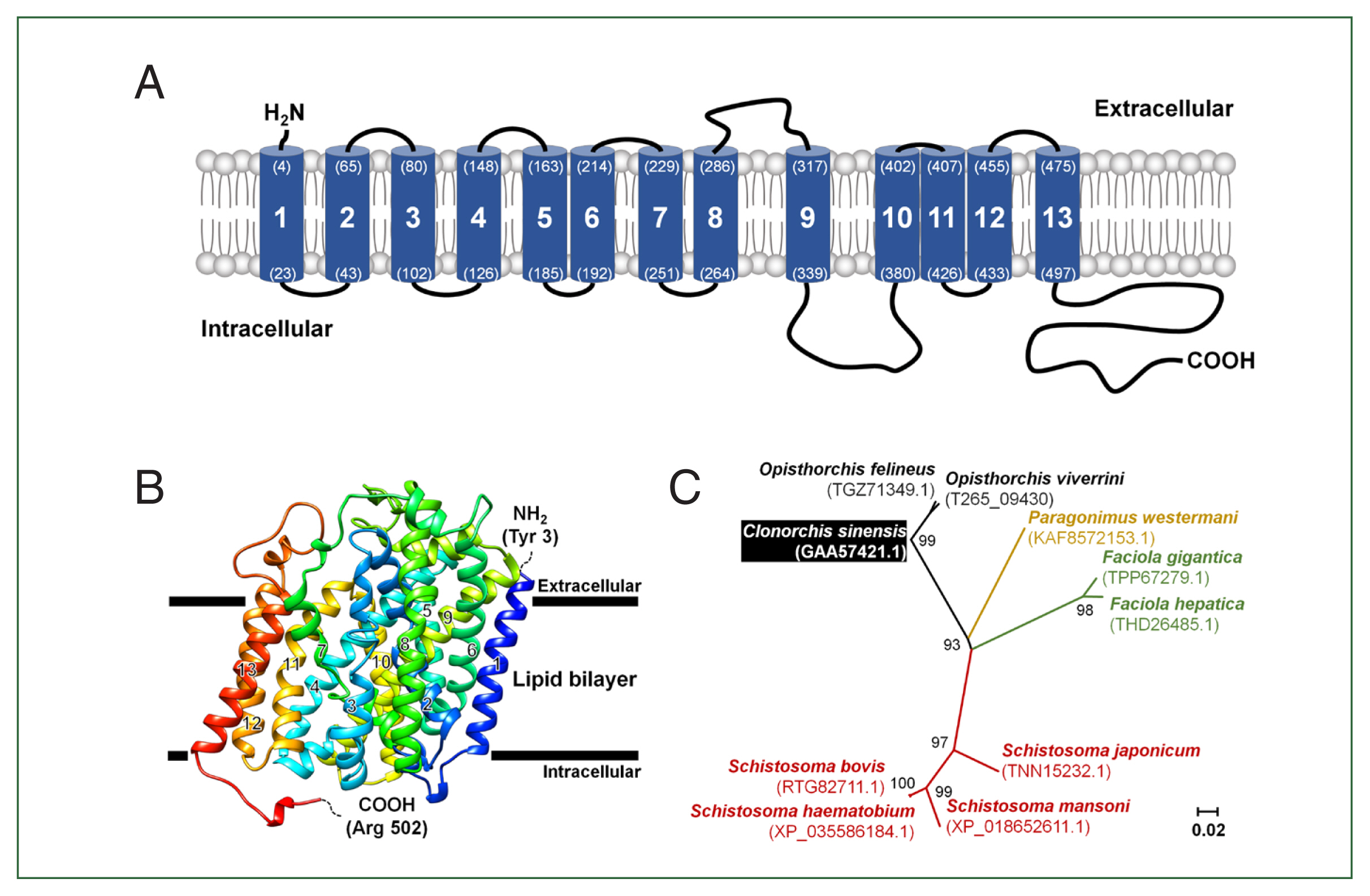
Fig. 2Uptake of various 3H- or 14C-labeled compounds by CsChT-expressing oocytes. The uptake rates of radiolabelled compounds were measured in water-injected oocytes (Control, white histogram) and CsChT-expressing oocytes (black histogram) for 1 h (mean±SE, n=8–10). The concentrations of substrates were as follows; [3H] choline, 10 μM; [3H] arginine, 100 nM; [14C] α-ketoglutarate, 5 μM; [14C] p-aminohippurate, 10 μM; [3H] taurocholate, 200 nM; [3H] estrone sulfate, 50 nM.
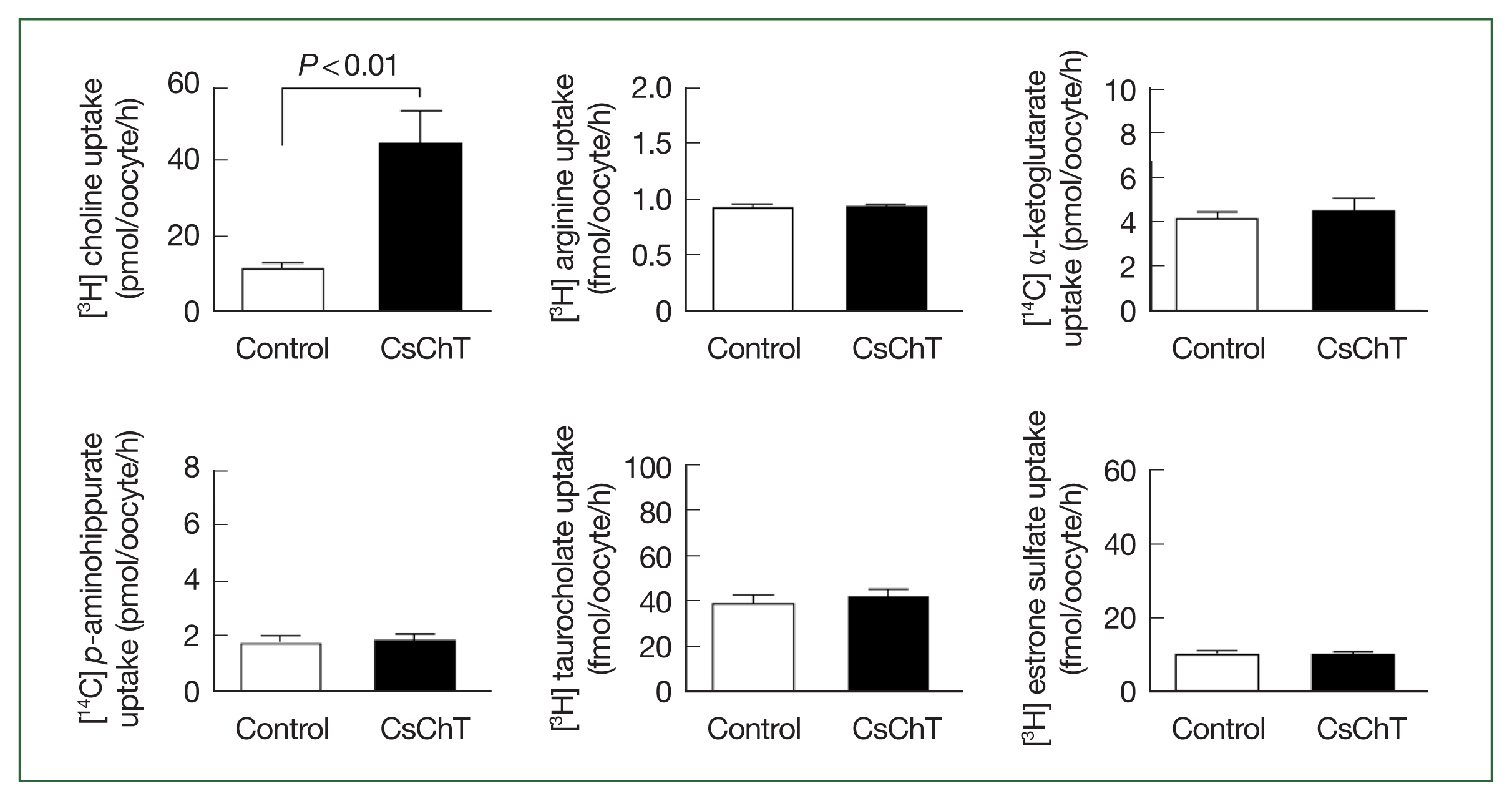
Fig. 3The transport properties of choline via CsChT. (A) The uptake of 10 μM [3H] choline in the CsChT-expressing oocytes or water-injected (control) oocytes was measured at the indicated CsChT expression times. (B) The uptake of 10 μM [3H] choline in control oocytes (open circle) and CsChT-expressing oocytes (closed circle) was measured at 15 min intervals up to 75 min. All results were represented by mean±SE (n=6–8).
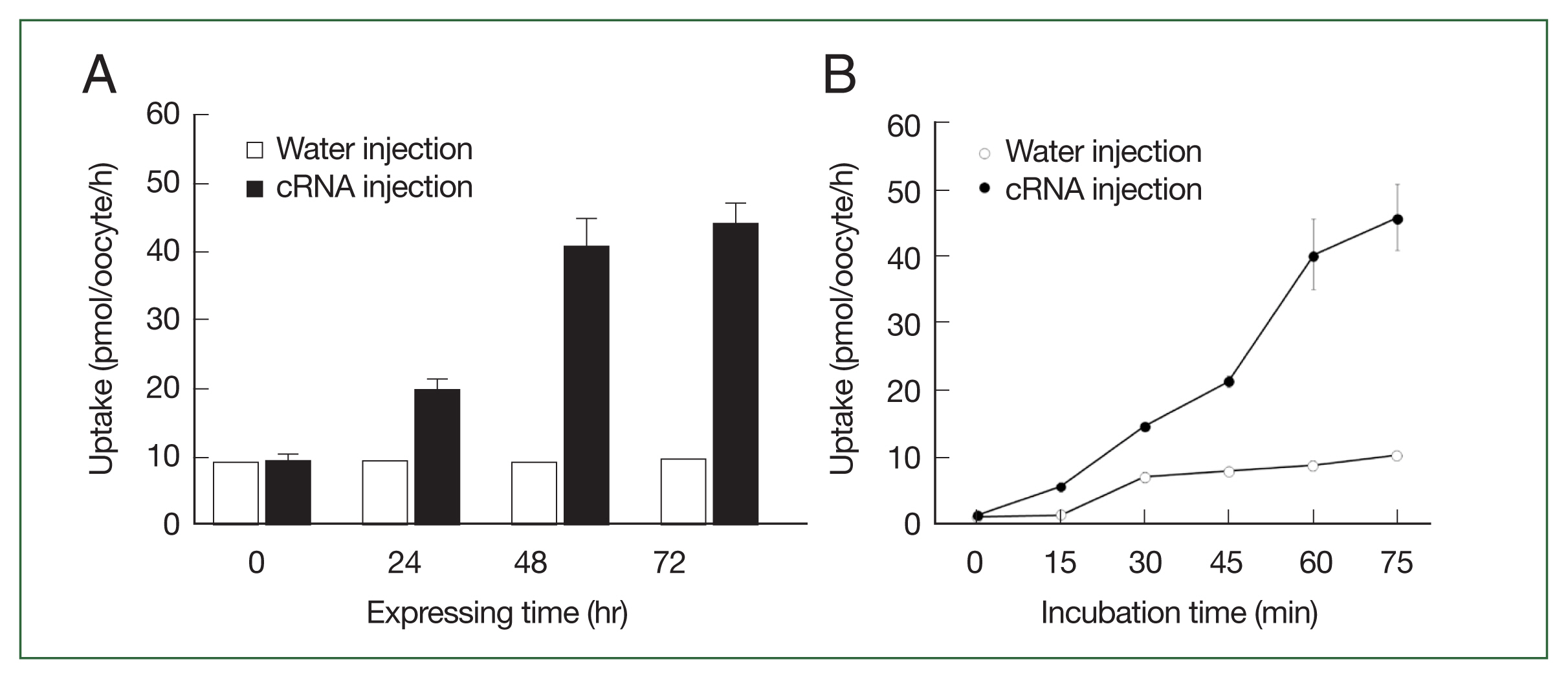
Fig. 4The sodium dependent transport properties of CsChT. (A) Effect of extracellular cation on [3H] choline uptake in CsChT-expressing oocytes. The uptake rate of [3H] choline (10 μM) was measured in the presence or absence of extracellular Na+. Extracellular Na+ was replaced with equimolar concentration of NMDG (N-methyl-d-glucosamine) and lithium (LiCl). The results were represented by mean±SE (n=6–8). (B) CsChT tertiary structure shows a putative Na+ binding pocket. The five Na+ binding amino acid residues are shown by sphere shapes, including Ala 59, Val 62, Ala 338, Ser 341, and Ser 342.

Fig. 5Trans-stimulatory effect and concentration dependence of CsChT-mediated uptake of [3H] choline. (A) The lack of a trans-stimulatory effect of choline on CsChT mediated efflux of choline was observed. Oocytes expressed with CsChT were incubated with 10 μM [3H] choline for 1 h and transferred to the ND96 solution (control) or ND96 containing 100 μM or 1,000 μM unlabelled choline. The efflux amount of choline during 1 h was shown as the percentage of the preloaded amount. (B) The uptake rates of choline by control (water-injected) or CsChT-expressing oocytes for 1 h were measured at variable concentrations (mean±SE, n=6–8). Inset, Lineweaver-Burk analysis of concentration dependent uptake of [3H] choline. V represents velocity; S represents the concentration of choline.
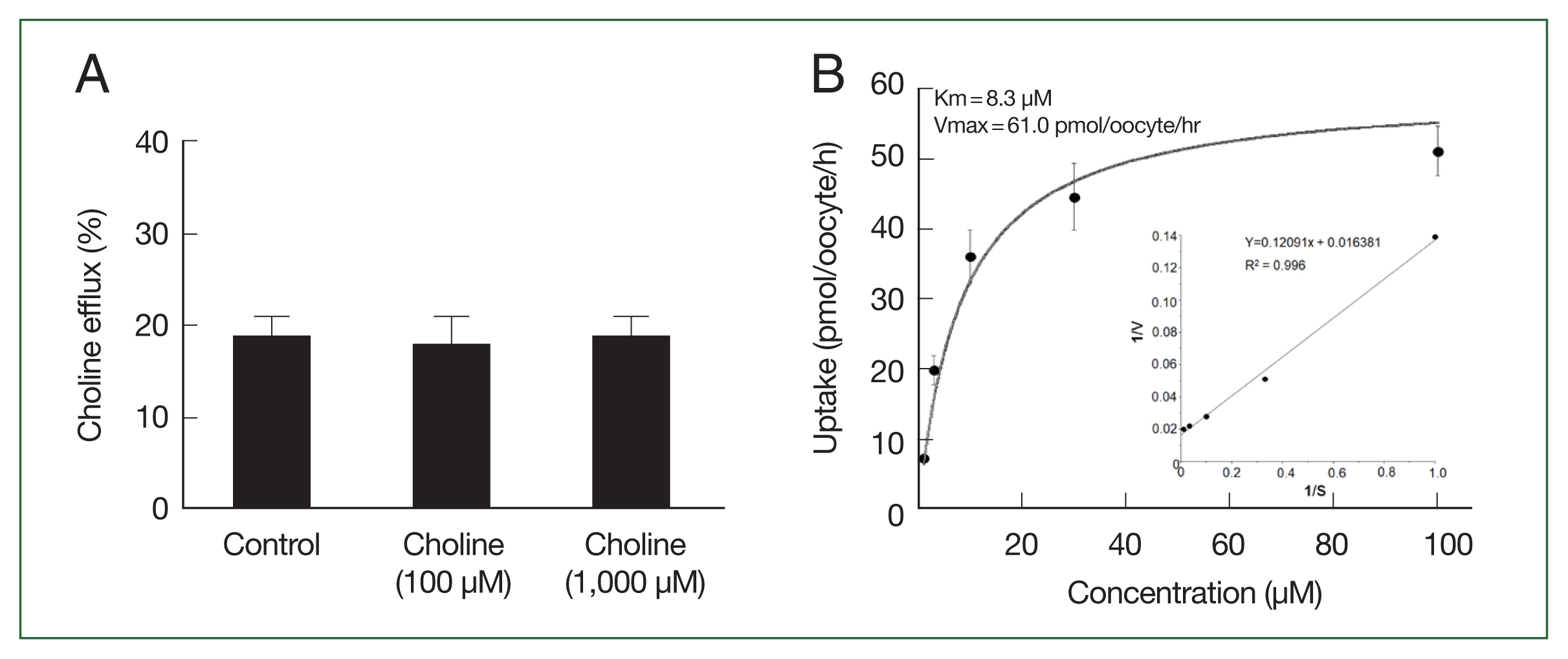
Table 1Amino acid identity percentages among the other related fluke proteins reported in the NCBI database
Table 1
|
Cs |
Fg |
Fh |
Of |
Pw |
Sb |
Sh |
Sj |
Sm |
|
Cs |
|
71 |
72 |
97 |
76 |
70 |
69 |
72 |
63 |
|
Fg |
|
|
95 |
71 |
71 |
69 |
68 |
70 |
63 |
|
Fh |
|
|
|
72 |
71 |
69 |
68 |
70 |
63 |
|
Of |
|
|
|
|
68 |
62 |
61 |
64 |
56 |
|
Pw |
|
|
|
|
|
69 |
69 |
71 |
64 |
|
Sb |
|
|
|
|
|
|
98 |
88 |
85 |
|
Sh |
|
|
|
|
|
|
|
87 |
84 |
|
Sj |
|
|
|
|
|
|
|
|
79 |
References
- 1. Kim EM, Kim JL, Choi SY, Kim JW, Kim S, et al. Infection status of freshwater fish with metacercariae of Clonorchis sinensis in Korea. Parasites Hosts Dis 2008;46(4):247-251.
https://doi.org/10.3347/kjp.2008.46.4.247
- 2. Qian MB, Chen YD, Liang S, Yang GJ, Zhou XN. The global epidemiology of clonorchiasis and its relation with cholangiocarcinoma. Infect Dis Poverty 2012;1(1):4.
https://doi.org/10.1186/2049-9957-1-4
- 3. Doanh PN, Nawa Y.
Clonorchis sinensis and Opisthorchis spp. in Vietnam: current status and prospects. Trans R Soc Trop Med Hyg 2016;110(1):13-20.
https://doi.org/10.1093/trstmh/trv103
- 4. Tang ZL, Huang Y, Yu XB. Current status and perspectives of Clonorchis sinensis and clonorchiasis: epidemiology, pathogenesis, omics, prevention and control. Infect Dis Poverty 2016;5(1):71.
https://doi.org/10.1186/s40249-016-0166-1
- 5. Rim HJ. Clonorchiasis: an update. J Helminthol 2005;79(3):269-281.
https://doi.org/10.1079/joh2005300
- 6. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, et al. A review of human carcinogens--part B: biological agents. Lancet Oncol 2009;10(4):321-332.
https://doi.org/10.1016/s1470-2045(09)70096-8
- 7. Park SK, Friedrich L, Yahya NA, Rohr CM, Chulkov EG, et al. Mechanism of praziquantel action at a parasitic flatworm ion channel. Sci Transl Med; 2021. 13(625):eabj5832
https://doi.org/10.1126/scitranslmed.abj5832
- 8. Robertson AP, Martin RJ. Ion-channels on parasite muscle: pharmacology and physiology. Invert Neurosci 2007;7(4):209-217.
https://doi.org/10.1007/s10158-007-0059-x
- 9. You H, Liu C, Du X, McManus DP. Acetylcholinesterase and nicotinic acetylcholine receptors in schistosomes and other parasitic helminths. Molecules 2017;22(9):1550.
https://doi.org/10.3390/molecules22091550
- 10. Hu Y, Xiao SH, Aroian RV. The new anthelmintic tribendimidine is an L-type (levamisole and pyrantel) nicotinic acetylcholine receptor agonist. PLoS Negl Trop Dis 2009;3(8):e499.
https://doi.org/10.1371/journal.pntd.0000499
- 11. Robertson AP, Puttachary S, Buxton SK, Martin RJ. Tribendimidine: mode of action and nAChR subtype selectivity in Ascaris and Oesophagostomum
. PLoS Negl Trop Dis 2015;9(2):e0003495.
https://doi.org/10.1371/journal.pntd.0003495
- 12. Ancelin ML, Torpier G, Vial HJ, Capron A. Choline incorporation by Schistosoma mansoni: distribution of choline metabolites during development and after sexual differentiation. J Parasitol 1987;73(3):530-535.
- 13. Guidi A, Petrella G, Fustaino V, Saccoccia F, Lentini S, et al. Drug effects on metabolic profiles of Schistosoma mansoni adult male parasites detected by 1H-NMR spectroscopy. PLoS Negl Trop Dis 2020;14(10):e0008767.
https://doi.org/10.1371/journal.pntd.0008767
- 14. Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J 2006;20(1):43-49.
https://doi.org/10.1096/fj.05-4707com
- 15. Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev 2009;67(11):615-623.
https://doi.org/10.1111/j.1753-4887.2009.00246.x
- 16. Michel V, Yuan Z, Ramsubir S, Bakovic M. Choline transport for phospholipid synthesis. Exp Biol Med 2006;231(5):490-504.
https://doi.org/10.1177/153537020623100503
- 17. Biagini GA, Ward SA, Bray PG. Malaria parasite transporters as a drug-delivery strategy. Trends Parasitol 2005;21(7):299-301.
https://doi.org/10.1016/j.pt.2005.05.013
- 18. Young BW, Podesta RB. Uptake and incorporation of choline by Schistosoma mansoni adults. Mol Biochem Parasitol 1985;15(2):105-114.
https://doi.org/10.1016/0166-6851(85)90112-4
- 19. Macedo JP, Schmidt RS, Maser P, Rentsch D, Vial HJ, et al. Characterization of choline uptake in Trypanosoma brucei procyclic and bloodstream forms. Mol Biochem Parasitol 2013;190(1):16-22.
https://doi.org/10.1016/j.molbiopara.2013.05.007
- 20. Giacomini KM, Yee SW, Koleske ML, Zou L, Matsson P, et al. New and emerging research on solute carrier and ATP binding cassette transporters in drug discovery and development: outlook from the international transporter consortium. Clin Pharmacol Ther 2022;112(3):540-561.
https://doi.org/10.1002/cpt.2627
- 21. Park JH, Kim MH, Sutanto E, Na SW, Kim MJ, et al. Geographical distribution and genetic diversity of Plasmodium vivax reticulocyte binding protein 1a correlates with patient antigenicity. PLoS Negl Trop Dis 2022;16(6):e0010492.
https://doi.org/10.1371/journal.pntd.0010492
- 22. Truant AL, Elliott SH, Kelly MT, Smith JH. Comparison of formalin-ethyl ether sedimentation, formalin-ethyl acetate sedimentation, and zinc sulfate flotation techniques for detection of intestinal parasites. J Clin Microbiol 1981;13(5):882-884.
https://doi.org/10.1128/jcm.13.5.882-884.1981
- 23. Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, et al. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem 2000;275(6):4507-4512.
https://doi.org/10.1074/jbc.275.6.4507
- 24. Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, et al. Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem 1999;274(19):13675-13680.
https://doi.org/10.1074/jbc.274.19.13675
- 25. Yamaoka K, Tanigawara Y, Nakagawa T, Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn 1981;4(11):879-885.
https://doi.org/10.1248/bpb1978.4.879
- 26. Ferguson SM, Bazalakova M, Savchenko V, Tapia JC, Wright J, et al. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc Natl Acad Sci USA 2004;101(23):8762-8767.
https://doi.org/10.1073/pnas.0401667101
- 27. Canty DJ, Zeisel SH. Lecithin and choline in human health and disease. Nutr Rev 1994;52(10):327-339.
https://doi.org/10.1111/j.1753-4887.1994.tb01357.x
- 28. Biagini GA, Pasini EM, Hughes R, De Koning HP, Vial HJ, et al. Characterization of the choline carrier of Plasmodium falciparum: a route for the selective delivery of novel antimalarial drugs. Blood 2004;104(10):3372-3377.
https://doi.org/10.1182/blood-2004-03-1084
- 29. Webb RA, Xue L. A novel Na+/HCO3--codependent choline transporter in the syncytial epithelium of the cestode Hymenolepis diminuta.
. Comp Biochem Physiol A Mol Integr Physiol 1998;119(2):553-562.
https://doi.org/10.1016/s1095-6433(97)00466-2
- 30. Saric J, Li JV, Wang Y, Keiser J, Bundy JG, et al. Metabolic profiling of an Echinostoma caproni infection in the mouse for biomarker discovery. PLoS Negl Trop Dis 2008;2(7):e254.
https://doi.org/10.1371/journal.pntd.0000254
- 31. Saric J, Li JV, Utzinger J, Wang Y, Keiser J, et al. Systems parasitology: effects of Fasciola hepatica on the neurochemical profile in the rat brain. Mol Syst Biol 2010;6:396.
https://doi.org/10.1038/msb.2010.49
- 32. Haga T. Molecular properties of the high-affinity choline transporter CHT1. J Biochem 2014;156(4):181-194.
https://doi.org/10.1093/jb/mvu047
- 33. Muramatsu I, Uwada J, Masuoka T, Yoshiki H, Sada K, et al. Regulation of synaptic acetylcholine concentrations by acetylcholine transport in rat striatal cholinergic transmission. J Neurochem 2017;143(1):76-86.
https://doi.org/10.1111/jnc.14127
- 34. Loffelholz K, Klein J, Koppen A. Choline, a precursor of acetylcholine and phospholipids in the brain. Prog Brain Res 1993;98:197-200.
https://doi.org/10.1016/s0079-6123(08)62399-7
- 35. Chai JY. Praziquantel treatment in trematode and cestode infections: an update. Infect Chemother 2013;45(1):32-43.
https://doi.org/10.3947/ic.2013.45.1.32
- 36. Lee JM, Lim HS, Hong ST. Hypersensitive reaction to praziquantel in a clonorchiasis patient. Korean J Parasitol 2011;49(3):273-275.
https://doi.org/10.3347/kjp.2011.49.3.273
- 37. Siqueira LDP, Fontes DAF, Aguilera CSB, Timoteo TRR, Angelos MA, et al. Schistosomiasis: drugs used and treatment strategies. Acta Trop 2017;176:179-187.
https://doi.org/10.1016/j.actatropica.2017.08.002
- 38. Xu LL, Jiang B, Duan JH, Zhuang SF, Liu YC, et al. Efficacy and safety of praziquantel, tribendimidine and mebendazole in patients with co-infection of Clonorchis sinensis and other helminths. PLoS Negl Trop Dis 2014;8(8):e3046.
https://doi.org/10.1371/journal.pntd.0003046
- 39. Xiao SH, Xue J, Xu LL, Zhang YN, Qiang HQ. Comparative effect of mebendazole, albendazole, tribendimidine, and praziquantel in treatment of rats infected with Clonorchis sinensis.
. Parasitol Res 2011;108(3):723-730.
https://doi.org/10.1007/s00436-010-2187-1
- 40. Okuda T, Nomura Y, Konishi A, Misawa H. Competitive inhibition of the high-affinity choline transporter by tetrahydropyrimidine anthelmintics. Eur J Pharmacol 2021;898:173986.
https://doi.org/10.1016/j.ejphar.2021.173986