Abstract
During the Edo period, Hachinohe Castle served as the residence of the Nanbu clan, the lords of the Hachinohe domain, and simultaneously functioned as the local government office. Although an analytical company reported on the soil samples from toilet remains within the castle, this study conducted a new analysis. Not only were Trichuris trichiura eggs found in Layer 21, but Metagonimus yokogawai and Dibothriocephalus nihonkaienesis eggs were also present. In Layer 20, which was initially thought to be free of parasitic organisms, T. trichiura, Ascaris lumbricoides, and M. yokogawai eggs were discovered. This paper discusses the differing results from previous studies, which demonstrate that the analytical methodology of Japanese archaeoparasitology is not yet well established, and suggests ways to improve it.
-
Key words: Archaeoparasitology, paleopathology, physical anthropology, food parasitology, host-parasite interactions
Parasite eggs excavated from ancient sites are excellent samples for investigating the eating habits and health of historic peoples. Parasites are host selective, meaning the type of diet and cooking methods can be inferred with a high probability from their presence, which is determined primarily from the types of parasite eggs found in stools. Studies of parasite eggs also provide important information on paleopathology and health during that period. In Japan, archaeological parasitology (archaeoparasitology) was established by Kanehara Masaaki, Kanehara Masako, and Matsui Akira in the 1990s and 2000s [
1,
2]. They examined the remains of the toilet at Heijo Palace, Koro-kan, Akita Castle, and other sites, where they discovered the eggs of
Ascaris lumbricoides,
Trichuris trichiura,
Clonorchis sinensis,
Metagonimus yokogawai, and
Taenia spp. [
2]. With the identification of those parasites, the diet of the people who lived and worked in these sites was reconstructed. These findings reflected the Japanese practice of using human feces fertilizer since agriculture began during the Yayoi period (c. 700 BCE–300 CE), a practice that continued until after World War II [
3].
T. trichiura and
A. lumbricoides adhere to vegetable matter, and their presence in human feces thus indicates the presence of such material. Thus, parasite eggs isolated from toilet remains are an important source of information on cultural transformations.
The scholarly contributions of archaeoparasitology have continued to the present day. Parasite egg analysis is necessary for understanding the soil samples thought to be from historical latrines, and many companies exist that perform such analyses. One such company previously conducted archaeoparasitological analysis on the ancient toilet remains at the site of Hachinohe Castle. However, upon obtaining soil samples from the same site and reexamining them, the writers of this paper found that the initial report was inaccurate in several respects. Researchers involved in archaeological parasitology must urgently clarify what needs to be done to prevent such inaccurate analyses. The purpose of this paper is to clarify the current state of archaeoparasitology in Japan and to show that, with sufficient analysis, it is possible to infer the paleopathology of historical peoples, their nutrition, parasitic infections, and the impact of those infections on their health.
Hachinohe Castle was the residence of the Hachinohe Nanbu clan and served as the fortress and government office of the Hachinohe domain from 1664 to 1871.
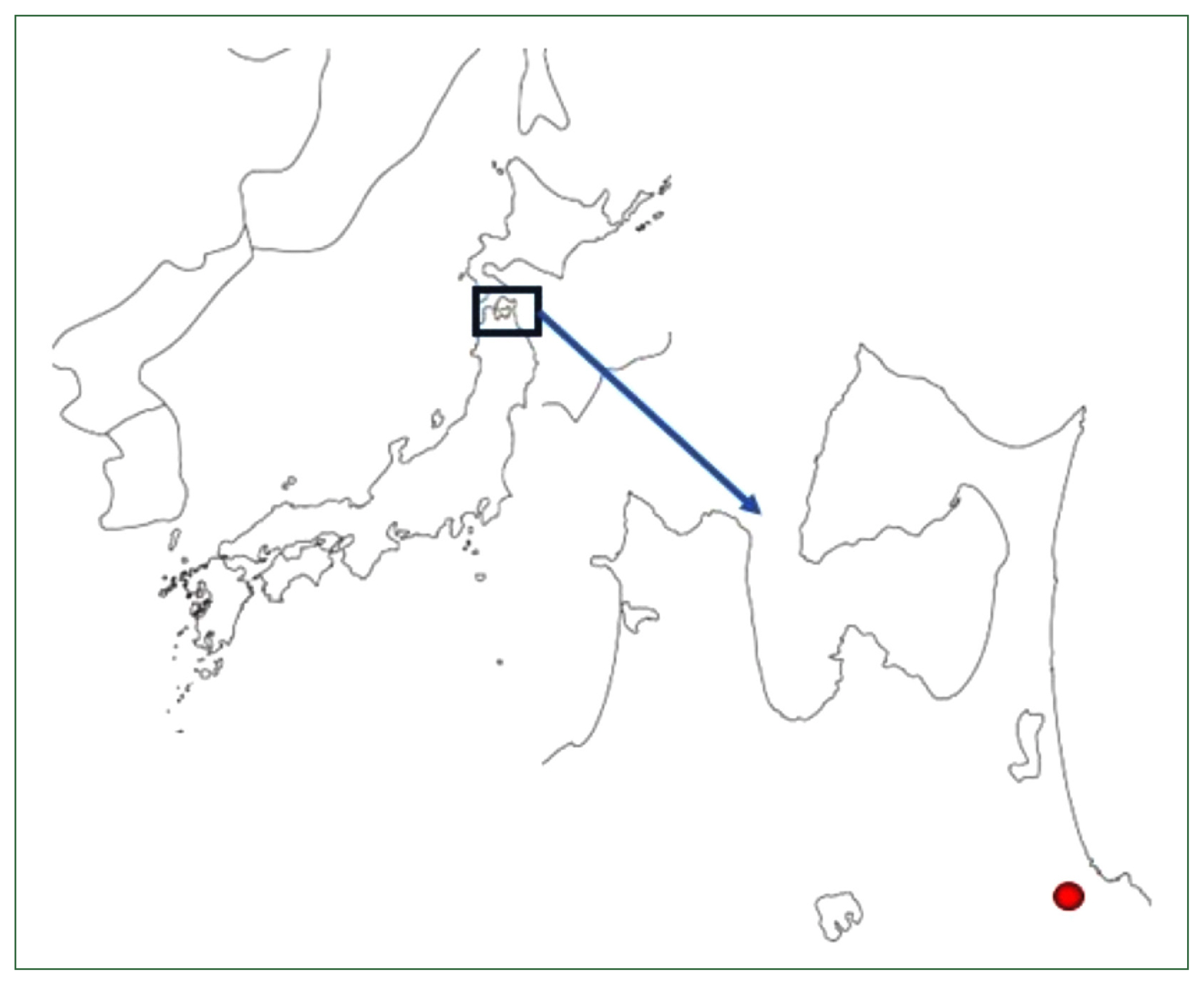
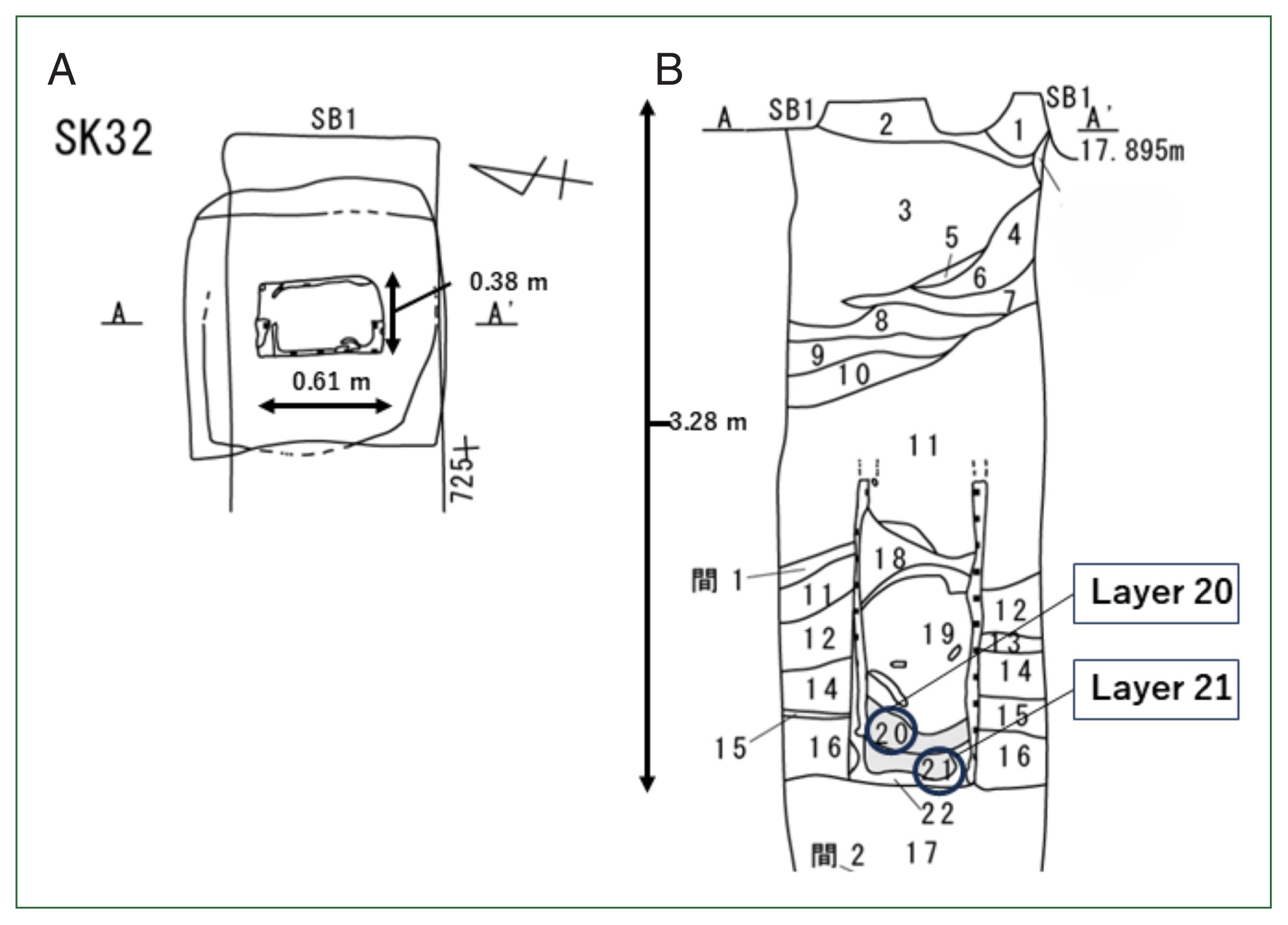
Fig. 1 shows the map of Hachinohe City in Aomori Prefecture; Hachinohe Castle was in the center of the city. It was initially reported that over 500
T. trichiura eggs were found in a clay pit, designated
Fig. 2 [
4].
Fig. 2 shows the upper view of the toilet remains (A) and their stratigraphy (B). The previous analysis also found that many
T. trichiura eggs were detected in Layer 21 of this stratigraphy, while no parasite eggs were detected in Layer 20.
In the present study, the authors reexamined the findings from the same pit using soil samples from Layers 20 and 21. Furthermore, this study estimated the eating habits of Hachinohe City residents from the parasite eggs and investigated the potential impact of those parasites on historical people’s health during the Edo period (1603–1868). The soil materials were housed at the Korekawa Archaeological Institution at the Hachinohe City Center for Archaeological and Cultural Properties. A large quantity remained of Layer 20, in which no parasite eggs had been previously found, but Layer 21 had been depleted for various analyses because parasite eggs had been detected in the previous study [
4]. One author (HF) visited the Korekawa Archaeological Institution and received samples of both layers. These samples were removed with a small disposable spoon onto a piece of medicine paper and their mass was measured: 2.46 g of residue from Layer 21 and 4.34 g of soil from Layer 20.
The soil sample from Layer 21 was solidified, probably due to the presence of various chemicals, so it was wrapped in medicine paper and softly crushed from the top with a pestle into small pieces. Each sample was then placed in a 15-mL centrifuge tube, which was then filled approximately 2/3 with sucrose water of specific gravity 1.3. Each sample was then vibrated with a vortex mixer for 15 min to separate the parasite eggs from the soil. Each centrifuge tube was then filled with more sucrose water until surface tension was generated, after which it was allowed to stand for 30 min. Then, a cover glass (18 mm×18 mm) was placed on top of each centrifuge tube with tweezers and allowed to stand for 30 min [
5]. Next, a small drop of Hoyer’s solution was added as an encapsulant and the cover glass was placed on a preparate and allowed to dry for 28 days. The prepared preparate was observed in transmission mode with an Olympus BX50 microscope (Olympus, Tokyo, Japan) and photographed with a WRAYCAM-noa2000 microscope camera (WRAYMER, Osaka, Japan) if necessary.
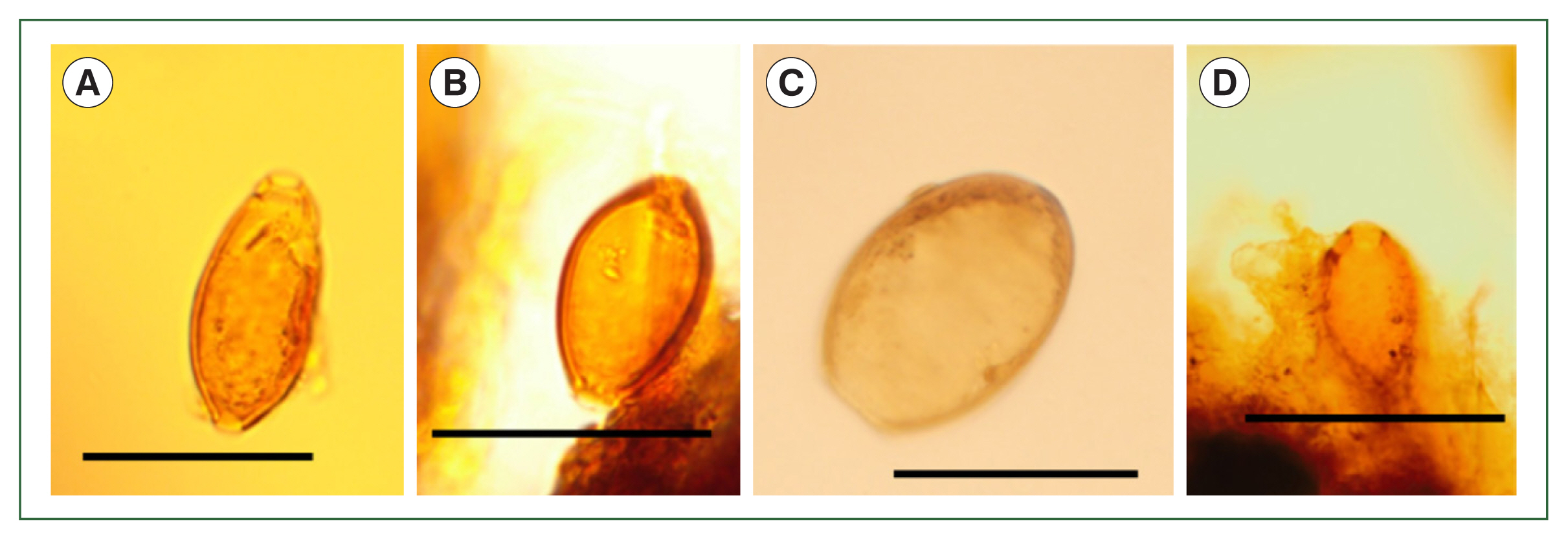
First, we will discuss Layer 21, where
T. trichiura eggs had been previously found [
4]. The residue of a relatively large number of
T. trichiura eggs were detected (
Fig. 3A, B). In addition, other parasite eggs were found, which were confirmed to be from
Dibothriocephalus nihonkaiensis (
Fig. 3C) and
M. yokogawai (
Fig. 3D). The previous report [
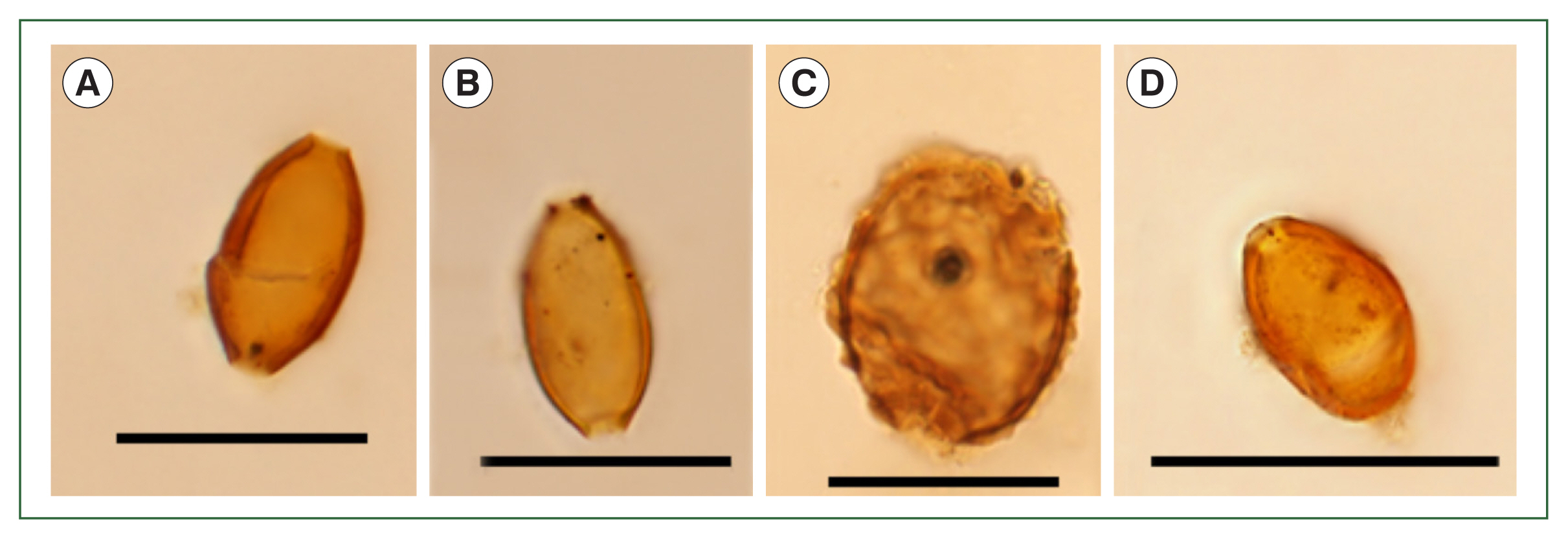
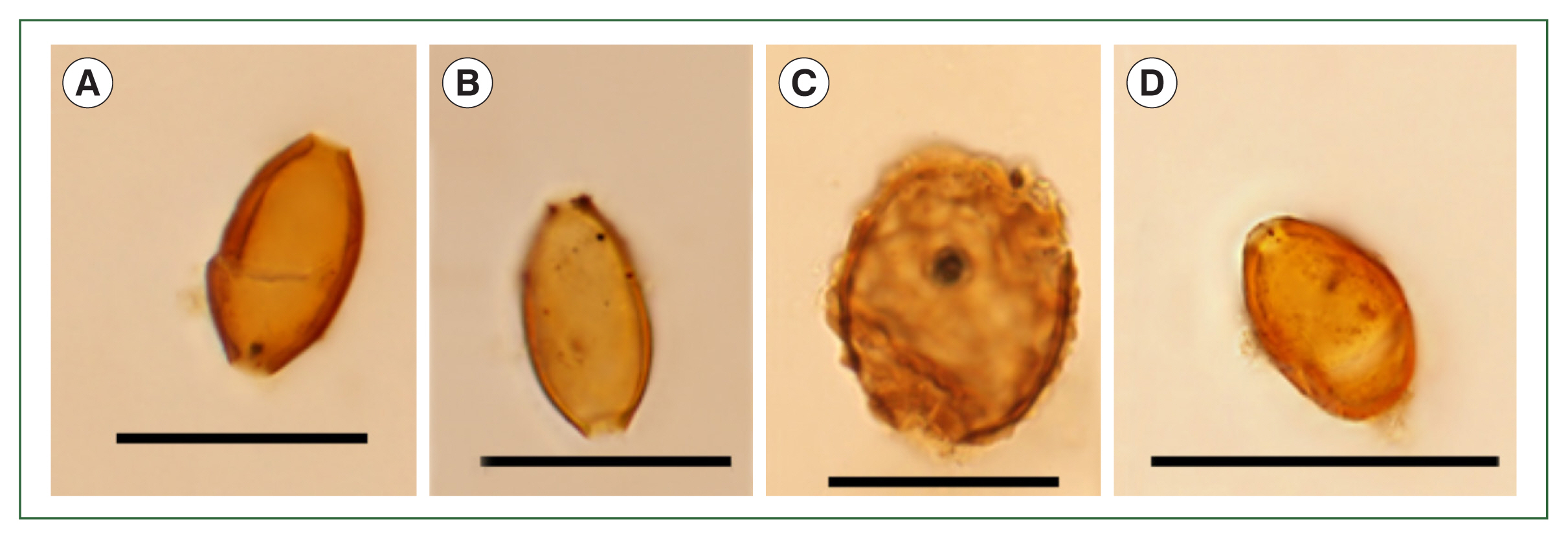
4] found no parasite eggs in Layer 20, but in this study, it was found to contain parasite eggs identified as being from
T. trichiura (
Fig. 4A, B),
A. lumbricoides (
Fig. 4C), and
M. yokogawai (
Fig. 4D). This layer was found to be a fecal sediment layer in previous studies [
6–
8]. In contrast, previous studies [
4] had found only
T. trichiura eggs.
The analysis of Layer 20, in which no parasite eggs were found in the previous study [
4], revealed eggs from
T. trichiura,
A. lumbricoides, and
M. yokogawai [
6,
7]. The fact that those parasites’ eggs were found in a small amount of soil (approximately 2.46 g and 4.34 g from Layers 21 and 20, respectively) suggests that like the soil deposited in the upper part of Layer 21, Layer 20 may be also from fecal remains. This is suggested by the fact that parasite eggs were not detected at all in several soil samples that were determined not to be toilet remains [
5]. It is thought that either Layer 20 came from an environment in which human feces were mixed with the soil, or that it was overlooked in previous reports but was in fact used as a toilet [
9].
The discovery of parasite eggs that had been missed in previous analyses indicates several problems with current Japanese archaeoparasitology practices. First, a wide variety of parasite eggs had been missed due to oversights on the part of the analyst(s). This can be a major problem in archaeological parasite analysis because it leads to missing information that could otherwise be obtained, such as the diet and health of historic peoples. It should be noted, however, that parasite eggs may not be detected from soil from toilet remains if the quantities analyzed are too small (meaning that it is not a problem of the analyst(s)). Second, to ensure the academic competence of archaeoparasitology analysts, people in leadership positions should encourage analysts to present their work at conferences and publish papers. In other words, authorities should make every effort to improve and update analysts’ knowledge. Third, analysts should be given sufficient time to clarify unclear data. A rushed, deadline-driven analysis can often result in a cursory analysis. It will not contribute to the future meaningful development of archaeology and anthropology. Finally, this issue should not be considered the responsibility of any specific individual, research institution, board of education, or analysis company, but rather one where all organizations and individuals involved in research should be fully aware of their responsibilities.
Archaeoparasitology can provide concrete indications of the dietary habits of people from the past and can uncover a great deal of information from a paleopathological perspective, such as the health hazards caused by parasites. The following is a brief description of the information obtained from the present re-examination. First, paleopathological considerations indicate that the presence of a small number of
T. trichiura does not lead to symptoms, but higher numbers can cause abdominal pain, diarrhea, hemorrhage, anemia, and potential death [
10].
A. lumbricoides roundworms live in the ileum and generally cause occasional abdominal pain, diarrhea, undernutrition, or hyperanesthesia. However, if many
A. lumbricoides become entangled in a mass and cause intestinal obstruction, they may invade the pancreatic duct and appendix, causing intestinal obstruction [
10]. When
A. lumbricoides stray into the stomach, severe gastric spasm, peritonitis due to intestinal perforation, and liver abscess due to hepatic perforation are known to occur and can be fatal [
10].
M. yokogawai in small numbers cause few symptoms, but when many are present, diarrhea, abdominal pain, and chronic inflammation of the ileum may occur [
10].
D. nihonkaiensis causes abdominal pain, diarrhea, abdominal distention, nausea, generalized morphology, and dizziness [
10]. These parasite infections are now almost completely curable in the early stages. In the Edo period, however, knowledge of parasitic disease was limited, and people with these infections often developed the above pathological conditions. In addition, it is likely that persistent diarrhea and anemia caused malnutrition, which in turn contributed considerably to a high mortality rate [
11–
13].
In the future, careful, high-level analysis in archaeological parasitology must be ensured. This requires multiple analyses, sufficient training for analysts, and ensuring sufficient time for analysis. Given the current state of archaeoparasite analysis in Japan, the often-unsatisfactory reporting of information and the resulting loss of valuable data must be remedied as soon as possible. Addressing these issues will help resolve the urgent need to bring Japanese archaeoparasitology to an internationally recognized level.
Notes
-
Author contributions
Conceptualization: Fujita H
Data curation: Fujita H, Funaba M
Formal analysis: Fujita H, Funaba M
Funding acquisition: Fujita H
Investigation: Fujita H
Project administration: Fujita H, Funaba M, Fujisawa SO
Writing – original draft: Fujita H, Fujisawa SO
-
Conflict of interest
The authors declare no conflict of interest related to this study.
-
Acknowledgments
This research was supported by Grant-in-Aid for Scientific Research (22H00020), Japan (KAKENHI).
Fig. 1A map of Aomori Prefecture, including Hachinohe City (indicated in red). Aomori Prefecture is the northernmost prefecture of the island of Honshu. Hachinohe Castle was located 20 m above sea level in the center of the city.
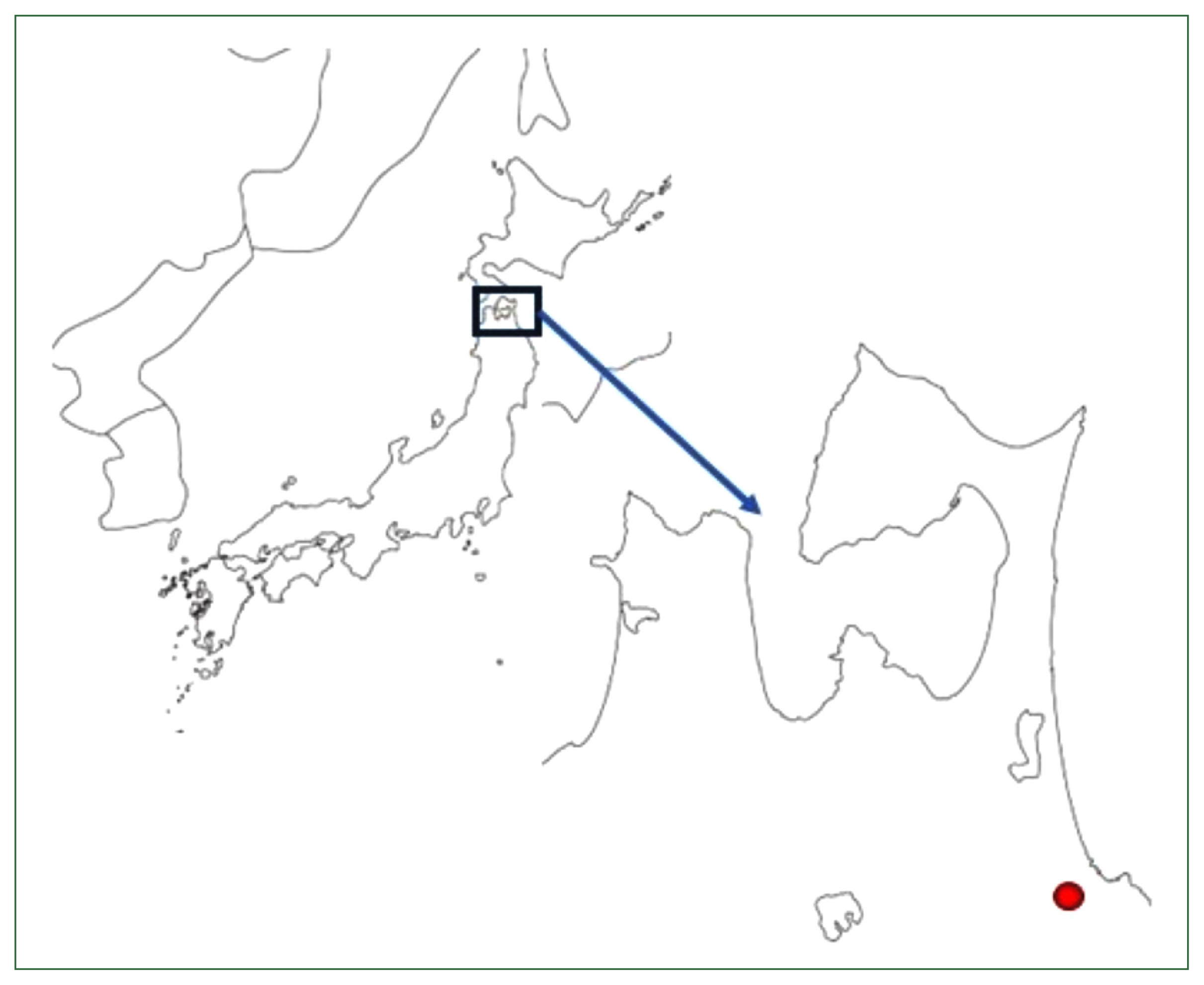
Fig. 2The details of sampling places in the toilet remains of Hachinohe Castle. The re-examination for this study was done in Layers 20 and 21. (A) The upper view of toilet remains, (B) the stratigraphy of the toilet remains.
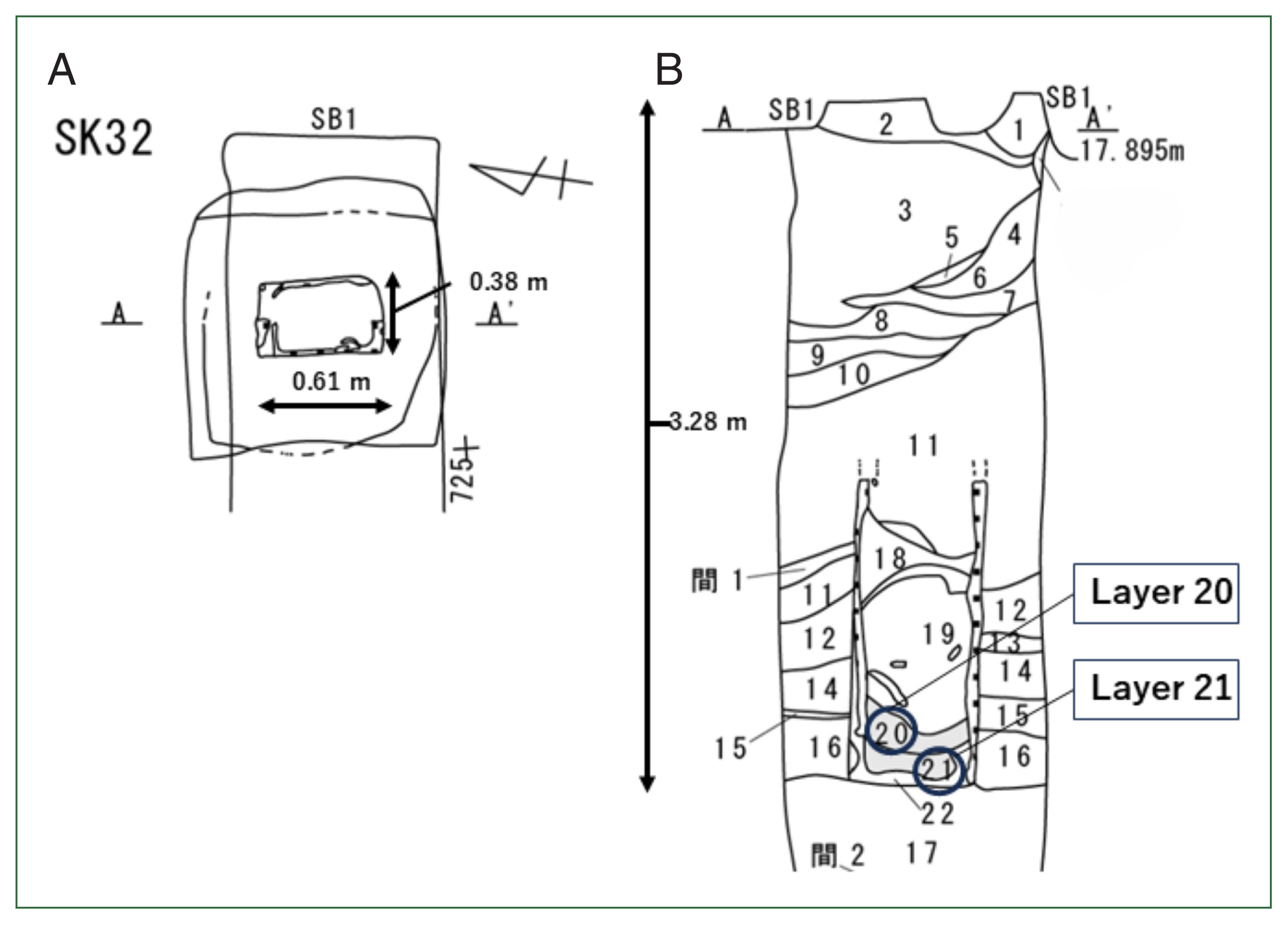
Fig. 3Microscopic examination of the samples in Layer 21. (A, B) Trichuris trichiura, (C) Dibothriocephalus nihonkaiensis, (D) Metagonimus yokogawai. Scale bar=50 μm.
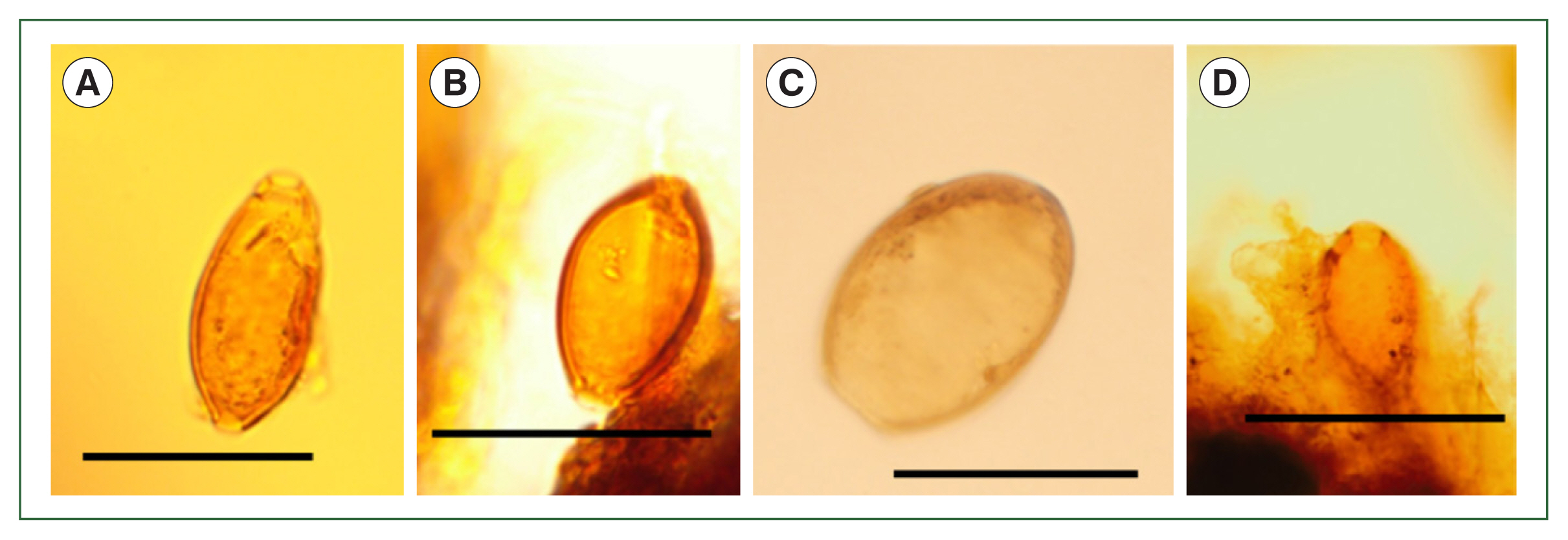
Fig. 4Microscopic examination of the samples in Layer 20. (A, B) Trichuris trichiura, (C) Ascaris lumbricoides, (D) Metagonimus yokogawai. Scale bar=50 μm.
References
- 1. Kanehara M, Kanehara M. Visual presentation of data from archaeological sediment. J Jpn Soc Vis Inf Stud 1994;14(53):9-14. https://doi.org/10.3154/jvs.14.79
- 2. Matsui A, Kanehara M, Kanehara M. Paleoparasitology in Japan: discovery of toilet features. Mem Inst Oswaldo Cruz 2003;98(Suppl 1):127-136. https://doi.org/10.1590/S0074-02762003000900019
- 3. Tada I. Parasite controls in Japan with emphasis on special characteristics. Trop Med Health 2008;36(Suppl 3):S49-S67. https://doi.org/10.2149/tmh.3SUPPLEMENT36-S49
- 4. Funaba M, Kana S, Tanaka Y, Shibaguchi R, Saito T, et al. Toilet feature detected from Hachinohe-jo castle. Bull Korekawa Archaeol Inst 2014;3:18-28.
- 5. Fujita H, Harihar S, Kumagai H, Shi DH. The problems for archaeoparasitology. The 77th Annual Meeting of the Anthropological Society of Nippon. 2023 Oct 8; Sendai, Japan.
- 6. Oh CS, Shim SY, Kim Y, Hong JH, Chai JY, et al. Helminth eggs detected in soil samples of possible toilet structure found at the capital area of ancient Baekje Kingdom of Korea. Korean J Parasitol 2021;59(4):393-397. https://doi.org/10.3347/kjp.2021.59.4.393
- 7. Kim J, Seo M, Fujita H, Chai JY, Park JW, et al. A parasitological study on the possible toilet ruins of the Japanese colonial period in Korea. Parasites Hosts Dis 2023;61(2):198-201. https://doi.org/10.3347/PHD.23013
- 8. Mitchell PD. Ancient parasite analysis: exploring infectious diseases in past societies. J Archaeol Sci 2024;170:106067. https://doi.org/10.1016/j.jas.2024.106067
- 9. Fujita H, Hong J, Shin D. Toilet archeology and archaeoparasitology. Anthropol Sci Jpn Ser 2023;131(1):9-15. https://doi.org/10.1537/asj.230217
- 10. Yoshida Y, Arizonon I. llustrated Human Parasitology. 9th ed. Nanzando. Tokyo, Japan. 2018, pp 91-193.
- 11. Marie C, Petri WA Jr, Muzny CA. Trichuriasis: MSD manual professional version [Internet]; 2025. [cited 2025 Apr 1]. Available from: https://www.msdmanuals.com/professional/infectious-diseases/nematodes-roundworms/trichuriasis
- 12. Marie C, Petri WA Jr, Muzny CA. Ascariasis: MSD manual professional version [Internet]; 2025. [cited 2025 Apr 1]. Available from: https://www.msdmanuals.com/professional/infectious-diseases/nematodes-roundworms/ascariasis
- 13. Shin DH, Fujita H, Hong JH. Urbanization and parasitic infections in the history of humans. Q Archaeol Suppl 2023;44:23-32.
Citations
Citations to this article as recorded by