Abstract
This experiment was focused on the characterization of anti-Toxoplasma monoclonal antibodies (mAbs) and the effect of mAbs on the parasite invasion of mouse peritoneal macrophages. Twenty eight mAbs including M110, M556, R7A6 and M621 were characterized by Ab titer, immunoglobulin isotyping and western blot pattern. Antibody titer (optical density) of 4 mAbs, M110, M556, R7A6 and M621, were 0.53, 0.67, 0.45 and 0.39 (normal mouse serum; 0.19) with the same IgG1 isotypes shown by Enzyme-linked immunosorbent assay (ELISA). Western blot analysis showed that M110, M556, R7A6 and M621 reacted with the 33 kDa (p30), 31 kDa (p28), 43 kDa and 36 kDa protein. Immunogold labelling of mAbs M110, M556, R7A6 and M621 reacted with the surface membrane, dense granules and parasitophorous vacuolar membrane (PVM), rhoptries and cytoplasm of tachyzoite, respectively. For in vitro assay, preincubation of tachyzoites with four mAbs, M110, M556, R7A6 and M621 resulted in the decrease of the number of infected macrophages (p < 0.05) and the suppression of parasite multiplication at 18 h postinfection. Four monoclonal antibodies including M110 (SAG1) were found to have an important role in the inhibition of macrophage invasion and T. gondii multiplication in vitro, and these mAbs may be suitable for vaccine candidates, diagnostic kit and for chemotherapy.
-
Key words: Toxoplasma, antibodies, monoclonal, electron microscopy, macrophages
INTRODUCTION
Toxoplasma gondii (
T. gondii) is an obligate intracellular protozoa infecting humans and animals. Human toxoplasmosis causes serious symtoms like encephalomyelitis in immunosuppressed patients and infection in pregnant women may lead to abortion and hydrocephalus of fetus. Diagnosis of toxoplasmosis was performed by serological tests, immunoblot, demonstration of the parasite in the blood or tissue and molecular biologic tools (
Ogata et al., 1983;
Aubert et al., 2000). Several serological tests have been developed to detect specific antibodies to
T. gondii in humans and animals. One of the immunologic tests using specific monoclonal antibodies (mAbs) was shown to be useful in detecting specific
Toxoplasma antigens (
Bonhomme, 1990;
Sohn and Nam, 1999). The immunodeterminant proteins of
Toxoplasma would be responsible for the immune response in host tissue; therefore, the purification and the characterization of these antigens should prove to be of a great value for diagnostic purposes.
Host cell penetration by
T. gondii is a complex phenomenon which involves not only a motile parasite but also the secretion of parasite. The protein secretion occurs sequentially from micronemes, rhoptries and dense granules following the invasion of
T. gondii into the host cell (
Achbarou et al., 1991;
Carruthers and Sibley, 1997). Apically located rhoptries rapidly discharge materials and dense granules release their contents into vacuolar space after invasion is complete (
Morrissette et al., 1994). The secreted dense granular proteins into the parasitophorous vacuole (PV) and parasitophorous vacuolar membrane (PVM) after the invasion changes the molecules of PVM and plays an important role for the multiplication of the parasite within the host cell and evasion of the host immune response (
Dubremetz and Schwartman, 1993;
Bonhomme, 1998). Many studies on the surface, cytoplasm, excretory and secretory antigens of
Toxoplasma have been carried out (
Johnson et al., 1983;
Charif et al., 1990;
Metsis et al., 1995).
This experiment was focused on the immunolocalization of the antigens in Toxoplasma RH tachyzoites at the ultrastructural levels and the effects of monoclonal antibodies on intracellular muliplication of T. gondii tachyzoites.
MATERIALS AND METHODS
Parasite
Toxoplasma gondii RH tachyzoites (106) were maintained by two weekly passages of tachyzoites to peritoneum of ICR mouse. Parasites were harvested in PBS from mouse peritoneum and host cells were removed by repeated low and high centrifuges (70 g for 3 min and 900 g for 10 min).
Monoclonal antibodies
Monoclonal antibodies against T. gondii were obtained by fusion of SP2/0 myeloma cells with the spleen cells of BALB/c mice immunized with soluble extract of T. gondii. Hybrid cells were cultured in HAT (Hypoxantine, Aminopterin, Thymidine in DMEM/20% FBS) medium for 2 weeks and the supernatants were tested for antibody titer which was then isotyped by ELISA method (Sigma, USA). The specific antigens recognized by monoclonal antibodies were chatacterized by SDS-PAGE/immunoblotting. Hybrid cells producing mAbs were injected to nude/SPF mouse intraperitoneally and after 2 weeks ascitic fluid was collected and kept at -20℃. The ascitic fluid was partially purified by a chromatography employing protein A Sepharose (Pharmacia-LKB, Uppsala, Sweden). In this experiment, four mAbs, M110, M556, R7A6 and M621 were used for immunoelectron microscopy and assay for inhibition of Toxoplasma invasion.
Antibody titer and isotyping by ELISA
Wells of microtiter plates were coated with 100 µl of Toxoplasma soluble antigen (5 µg/ml) in 0.5 M coating buffer (pH 7.2). After washing, mouse ascites (hybrid cell injected) diluted in PBS-Tween (1:200) were added. The wells were washed and incubated with peroxidase-conjugated affinipure F(ab')2 fragment goat anti-mouse IgG (Jackson ImmunoResearch, PA, USA) diluted in PBS-Tween (1:1000). After washing, 100 µl of substrate solution (o-phenylendiamine dihydrochloride) containing 0.1% H2O2 was added and then the absorbance at 492 nm was measured using a sphectrophotometer (Dynex Technologies Inc. Va, USA).
Western blotting
SDS-PAGE (Sodium dodecyl sulfate - polyacrylamide gel electrophoresis) was performed according to Laemmli (
1970) and separated proteins were transferred onto nitrocellulose paper as described by Towbin et al. (
1979). Nitrocellulose strips were incubated overnight at 4℃ with mouse ascites from hybridomas diluted to 1:200 in PBS-5% skimmed milk. After washing, strips were incubated in affinipure F(ab')
2 fragment peroxidase conjugated goat anti-mouse IgG (Jackson ImmunoResearch, PA, USA) 1:200 diluted in PBS-5% skimmed milk and reacted with DAB (diaminobenzidine, Sigma) solution.
T. gondii were harvested from ICR mice with PBS after 48 (intracellular) and 96 hr (extracellular) after intraperitoneal inoculation. Para-sites were fixed in 4% paraformaldehyde, 0.05% glutaraldehyde in cacodylate buffer + 3% sucrose for 2 hr at the room temperature. After sequential dehydration with 20%, 50% and 70% ethanol, embedding in LR White (London Resin Co., Taab, England) was performed. Ultrathin sections on nickel grid were incubated with NH4Cl, 0.5 M Tris buffer, followed by 2 h incubation with mouse ascites of hybridoma (1:40) and incubated with colloidal gold (12 nm) conjugated goat anti-mouse IgG (1:40, Sigma). After washing, the section was stained with 4% uranyl acetate in water and observed with an electron microscope (Hitachi 600, Hitachi, Japan).
Infection of macrophages with mAb treated tachyzoites
T. gondii tachyzoites were obtained from the peritoneal cavity of ICR mice. They were washed twice in MEM and resuspended with equal volume of mAb solution (1:10) for 30 min at 37℃ (5 × 105 parasite/ml).
BALB/c mice were injected peritoneally with LPS (lipopolysaccharide 10 µg/0.1ml, Sigma) for 3 days prior to the isolation of macrophages into MEM/10% FBS from non-adherent cells on 12 well plate with cover glass. Macrophage monolayers (1 × 106) were washed with PBS and infected with Toxoplasma tachyzoites (macrophage: tachyzoite = 1:2). After incubation for 1 hr at 37℃ in a 5% CO2 incubator, the monolayers were washed with PBS to remove extracellular tachyzoites and reincubated for 4 hr and 18 hr. The cover glass was fixed in 100% methanol, stained with Giemsa solution and examined by light microscopy. The number of infected macrophages/300 macrophages and number of tachyzoites/100 infected macrophage on light microscope were evaluated.
RESULTS
Chracterization of T. gondii monoclonal antibodies
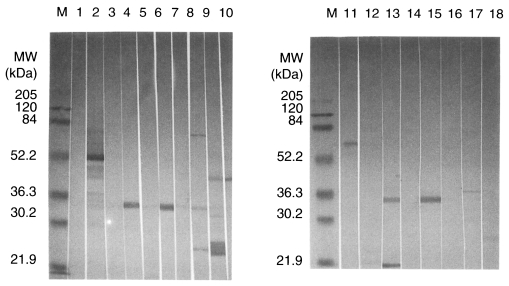
Antibody titers of 28 mAbs were determined by ELISA. The highest titer was 0.67 for M556 and the lowest titer was 0.25 for R6C2 (cut off value; 0.21). Isotype of mAbs against
T. gondii were IgG1, IgG2b and IgM. Specific antigens of
T. gondii were observed by 4 mAbs by western blotting (
Table 1;
Fig. 1).
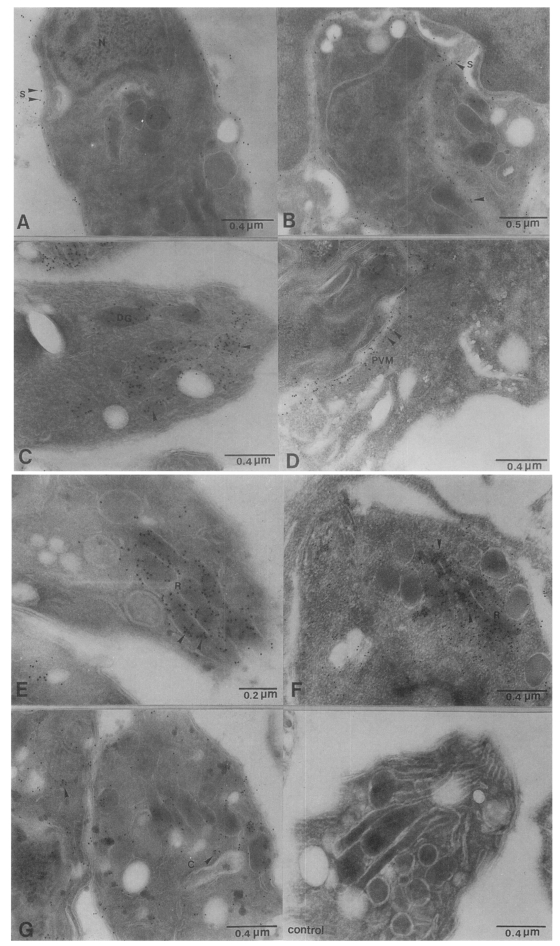
The ultrathin sections of intra- and extracellular
T. gondii treated with four mAbs, M110, M556, R7A6 and M621 were observed for immunolocalization. A thin section used as a control showed no or minute gold particle adherence. The M110 mAb revealed an intense staining with colloidal gold on the surface membrane of tachyzoite. Immunogold labelling of M556 showed the ultrastructural localization of the antigens in dense granules and PVM. Collodal gold labelling of R7A6 and M621 reacted with rhoptries and cytoplasm of
Toxoplasma tachyzoite, respectively (
Fig. 2).
Tachyzoites pretreated with mAbs (M110, M556, R7A6 and M621) were infected to mouse macrophages in 12 well culture plate. There were significant differences in the number of infected macrophages and the number of intracellular parasites per macrophage between mAb treated and untreated groups. Macrophage infection rates of M110, M556, R7A6 and M621 treated tachyzoites were 7.8%, 18.5%, 18.8% and 28.5%, respectively (control; 65.8%), at 18 hr after infection. Four mAbs, M110, M556, R7A6 and M621 mixed group showed 7.3% infection rate of macrophage at 18 h post-infection (P.I.) (
Table 2).
DISCUSSION
The purification of Toxoplasma antigens is valuable for many purposes and these antigens could play a major role in the protective immunity against toxoplasmosis. Using ELISA technique, mAbs showed high titers when the plate was coated with tachyzoite soluble antigens. We selected four mAbs for further studies, immunolocalization and protective role of mAb, in vitro. Antibody titers of mAbs, M110, M556, R7A6 and M621, were 0.53, 0.67, 0.45 and 0.39, respectively, in O.D. (normal mouse; 0.19) using ELISA. When IgG subclass responses to the antigens causing Toxoplasma infection were tested, IgG1 antibodies were the predominant subclass. According to the immunoblot analysis, the antigens recognized by M110, M556, R7A6 and M621 were the band patterns corresponding to 33 kDa (P30), 30 kDa (P28), 43 kDa and 36 kDa, respectively.
The surface of the
Toxoplasma tachyzoite is dominated by five major proteins including P30 (
Grimwood and Smith, 1992). The molecules located on the surface of the parasite are important to invade the host cell and SAG-1 (P30) plays a major role in invasion (
Couvreur et al., 1988;
Grimwood and Smith, 1995). Ultrastructural localization of antigenic protein of
T. gondii was examined by a ultrathin section using an immunogold technique with
Toxoplasma specific mAbs. The recognition of P30 (33 kDa) by mAb M110 demonstrated a homogenous distribution on the surface of intra- and extra-tachyzoites.
T. gondii has two main secretory organells, the rhoptries and the dense granules, that are involved in the invasion process. The secretory organelles are supposed to contain antigenic components that are discharged during enzymatic and metabolic processes and parasitophorous vacuole formation (
Morrissette et al., 1994). The mAb M556 immunolocalization in tachyzoites demonstrated in the dense granules and PV. The proteins in dense granules are secreted into the PV shortly after the host cell invasion and become associated with PVM or tubulovesicular network. Immunogold labeling of mAb R7A6 reacted with rhoptries in the anterior part of the parasite. The rhoptries secrete inner contents during the penetration and the secreted product lyses the host cell plasmalemma. The contents of the rhoptries are extruded during the early stages of the host cell invasion, and some of the proteins become associated with the formation of PVM (
Nichols et al., 1983;
Saffer, 1992;
Carruthers and Sibley, 1997).
Active invasion of
T. gondii into the host cell with apical complex occurs in nonphagocytic cells. During the active invasion of the parasite, tachyzoite invaginate the host cell membrane to form a parasitophorous vacuole (PV) that does not fuse with host lysosome and avoid parasite damage (
Huskinson et al., 1989;
Sibley, 1995).
However, the parasite-host cell interaction is more complicated in phagocytic cells since a competition occurs between the active invasion and the passive phagocytosis. Dead parasites or viable tachyzoites opsonized with specific antibodies are passively internalized by phagocytes or FcR-transfected fibroblast (
Charif, 1990). Binding of antibody-opsonized parasite to the host cell FcR receptors triggers phagocytosis and the parasite is internalized to the phagosome (
Fadul et al., 1995). The Fc receptor on
T. gondii affects the balance between invasion and phagocytosis (
Vercammen et al., 1999). When
Toxoplasma are treated with specific mAb before infection, the ability of parasite to multiply reduces in murine macrophage (
Hauser and Remington, 1981;
Mineo et al., 1994;
Vercammen et al., 1999). In this experiment, the invasion of
T. gondii tachyzoites into mouse peritoneal macrophage was inhibited after the pretreatment of polyclonal immune serum, mAb M110 and M110, M556, R7A6 and M621 mixed mAbs. It means that tachyzoites pretreated with mAbs were phagocytized by macrophages rather than active invasion of tachyzoite. In cases of polyclonal immune serum, M556, R7A6 and M621, they showed low levels of macrophage invasion at 4 hr, but at 18 hr P.I., tachyzoite multiplication increased to the level which is similar to the control group without serum treatment. However, mAb treated
Toxoplasma infected groups decreased cell lysis by parasite multiplication at 18 hr P.I. It was of interest to note that the addition of normal mouse serum to the tachyzoites suppressed macrophage invasion at 4 hr P.I. but sharply increased in the rate of invasion at 18 hr P.I. Antibodies against the secretion of rhoptries, dense granules and micronemes had no effect on the invasion shown in in vitro neutralization assay (
Grimwood and Smith, 1996). The major finding of this study is that mAb, M110, recognized the surface antigen of tachyzoite, P30 (33 kDa) and this mAb was a potent inhibitor of host cell invasion than other mAbs, M556, R7A6 and M621.
ACKNOWLEDGEMENT
This work was supported by the grant No. 1999-1-20200-002-2 from the Basic Research Program of the Korea Science & Engineering Foundation.
We would like to thank Professor Nam HW in Department of Parasitology, Catholic University of Korea, College of Medicine, for kindly supplying Toxoplasma gondii monoclonal antibodies, Dr. Kwon JG and Mr. Choi HK in Hanyang University, College of Medicine, for assisting electron microscopy.
References
- 1. Achbarou A, Mercereau-Puijalon O, Sadak A, et al. Differential targeting of dense granule proteins in the parasitophorous vacuole of Toxoplasma gondii. Parasitology 1991;103:321-329.
- 2. Aubert D, Maine GT, Villena I. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol 2000;38:1144-1150.
- 3. Bonhomme A, Boulanger F, Bharadwaj LM, et al. Toxoplasma gondii: Immunocytochemistry of four immunodominant antigens with monoclonal antibodies. Exp Parasitol 1990;71:439-451.
- 4. Bonhomme A, Maine GT, Beorchia A, et al. Quantitative Immunolocalization of a P29 protein (GRA7), a new antigen of Toxoplasma gondii. J Histochem Cytochem 1998;46:1411-1421.
- 5. Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol 1997;73:114-123.
- 6. Charif H, Darcy F, Torpier G, Cesbron-Delauw MF, Capron A. Toxoplasma gondii: Characterization and localization of antigens secreted from tachyzoites. Exp Parasitol 1990;71:114-124.
- 7. Couvreur G, Sadak A, Fortier B, Dubremetz JF. Surface antigens of Toxoplasma gondii. Parasitology 1988;97:1-10.
- 8. Dubremetz JF, Schwartzman JD. Sub-cellular organelles of Toxoplasma gondii and host cell invasion. Res Immunol 1993;144:31-33.
- 9. Fadul CE, Channon JY, Kasper LH. Survival of immunoglobulin G-opsonized Toxoplasma gondii in nonadherent human monocytes. Infect Immun 1995;63:4290-4294.
- 10. Grimwood J, Smith JE. Toxoplasma gondii: The role of a 30-kDa surface protein in host cell invasion. Exp Parasitol 1992;74:106-111.
- 11. Grimwood J, Smith JE. Toxoplasma gondii: Redistribution of tachyzoite surface protein during host cell invasion and intracellular development. Parasitol Res 1995;81:657-661.
- 12. Grimwood J, Smith JE. Toxoplasma gondii: The role of parasite surface and secreted proteins in host cell invasion. Int J Parasitol 1996;26:169-173.
- 13. Hauser WE, Remington JS. Effect of monoclonal antibodies on phagocytosis and killing of Toxoplasma gondii by normal macrophages. Infect Immun 1981;32:637-640.
- 14. Huskinson J, Stepick-Biek PN, Araujo FG, Thulliez P, Suzuki Y, Remington JS. Toxoplasma antigens recognized by immunoglobulin G subclasses during acute and chronic infection. J Clin Microbiol 1989;27:2031-2038.
- 15. Johnson AM, McDonald PJ, Neoh SH. Monoclonal antibodies to Toxoplasma cell membrane surface antigens protect mice from Toxoplasmosis. J Protozool 1983;30:351-356.
- 16. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680-685.
- 17. Metsis A, Pettsersen E, Petersen E. Toxoplasma gondii: Characterization of a monoclonal antibody recognizing antigens of 36 and 38 kDa with acid phosphatase activity located in dense granules and rhoptries. Exp Parasitol 1995;81:472-479.
- 18. Mineo JR, Khan IA, Kasper LH. Toxoplasma gondii: A monoclonal antibody that inhibits intracellular replication. Exp Parasitol 1994;79:351-361.
- 19. Morrissette NS, Bedian V, Webster P, Roos DS. Characterization of extreme apical antigens from Toxoplasma gondii. Exp Parasitol 1994;79:445-459.
- 20. Nichols BA, Chiappino ML, O'Connor GR. Secretion from the rhoptries of Toxoplasma gondii during host-cell invasion. J Ultrastruct Res 1983;83:85-98.
- 21. Ogata K, Arakawa M, Kasahara T, et al. Detection of Toxoplasma membrane antigens transferred from SDS-polyacrylamide gel to nitrocellulose with monoclonal antibody and avidin-biotin, peroxidase anti-peroxidase and immunoperoxidase methods. J Immunol Methods 1983;65:75-82.
- 22. Saffer LD, Mercereau-Puijalon O, Dubremetz JE, Schwartzman JD. Localization of a Toxoplasma gondii rhoptry protein by immunoelectron microscopy during and after host cell penetration. J Protozool 1992;39:526-530.
- 23. Sibley LD. Invasion of vertebrate cells by Toxoplasma gondii. Trends Cell Biol 1995;5:129-132.
- 24. Sohn WM, Nam HW. Western blot analysis of stray cat serum against Toxoplasma gondii and the diagnostic availability of monoclonal antibodies in sandwich-ELISA. Korean J Parasitol 1999;37:249-256.
- 25. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci 1979;76:4350-4354.
- 26. Vercammen M, Scorza T, Bouhdid AE, et al. Opsonization of Toxoplasma gondii tachyzoites with nonspecific immunoglobulins promotes their phagocytosis by macrophages and inhibits their proliferation in nonphagocytic cells in tissue culture. Parasite Immunol 1999;21:555-563.
Fig. 1Western blot of the antigens of Toxoplasma gondii recognized by anti-Toxoplasma monoclonal antibodies. M, marker; lane 1, normal mouse serum; lane 2, anti-T. gondii polyclonal mouse serum; lane 3-10, mAbs, M556, M563, M227, M110, M737, P415, M731 and R7A6; lane 11-18, mAbs, M695, M697, M217, M378, M577, M75, M621 and M505.
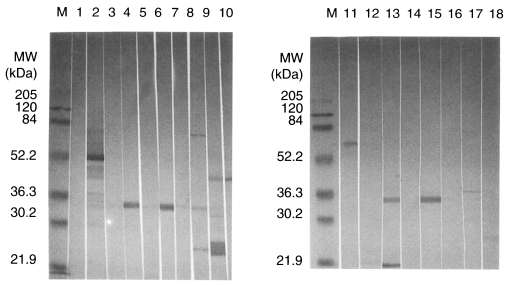
Fig. 2Colloidal gold immunoelectron micrographs reacting with intra- and extracellular Toxoplasma gondii tachyzoite (A & B) mAb, M110 located on the surface of T. gondii, (C & D) mAb, M556 was associated with dense granules and PVM, (E & F) mAb, R7A6 labelled in the rhoptries, (G) mAb, M621 was scattered in the cytoplasm of tachyzoite, (H) normal mouse serum reacted with T. gondii (N, nucleus; S, surface; DG, dense granules; R, rhoptries; C, cytoplasm of Toxoplasma; PVM, parasitophorous vacuolar membrane).
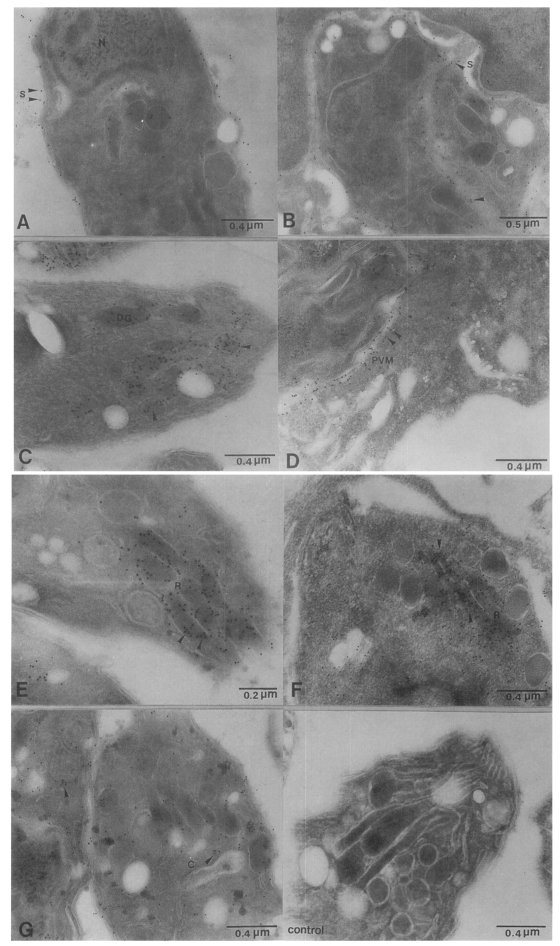
Table 1.Characterization of 28 Toxoplasma gondii monoclonal antibodies by ELISA and Western blot
Table 1.
|
Monoclonal antibody |
Ab. titer (O.D.)a)
|
Isotyping |
Molecular weight (kDa) |
|
M556 |
0.67 ± 0.03 |
IgG1 |
30 |
|
M563 |
0.61 ± 0.06 |
IgG1 |
33 |
|
M227 |
0.59 ± 0.01 |
IgG1 |
— |
|
M110 |
0.53 ± 0.04 |
IgG1 |
33 |
|
M737 |
0.49 ± 0.01 |
IgG1 |
— |
|
P415 |
0.46 ± 0.01 |
IgM |
74, 33, 25 |
|
M731 |
0.45 ± 0.01 |
— |
43, 32. 26-24 |
|
M247 |
0.45 ± 0.01 |
IgG2b |
ndb)
|
|
R7A6 |
0.45 ± 0.01 |
IgG1 |
43 |
|
M695 |
0.45 ± 0.03 |
IgM |
65 |
|
M697 |
0.44 ± 0.01 |
IgG1 |
— |
|
M217 |
0.42 ± 0.02 |
IgG1 |
35, 21 |
|
M378 |
0.41 ± 0.01 |
IgG1 |
— |
|
M577 |
0.40 ± 0.06 |
IgG1 |
35 |
|
M75 |
0.40 ± 0.02 |
IgG1 |
— |
|
M621 |
0.39 ± 0.06 |
IgG1 |
36 |
|
R1C4 |
0.36 ± 0.02 |
IgG1 |
nd |
|
M507 |
0.35 ± 0.01 |
IgG1 |
— |
|
M638 |
0.33 ± 0.01 |
IgG1 |
28 |
|
M505 |
0.32 ± 0.02 |
IgG1 |
26 |
|
M730 |
0.32 ± 0.02 |
IgG1 |
28, 25 |
|
M317 |
0.31 ± 0.01 |
IgM |
— |
|
R6A5 |
0.30 ± 0.01 |
IgG1 |
83 |
|
M536 |
0.30 ± 0.04 |
IgG1 |
— |
|
R4B5 |
0.30 ± 0.02 |
IgG1 |
83, 74, 43 |
|
M736 |
0.27 ± 0.01 |
IgM |
nd |
|
R5C1 |
0.25 ± 0.01 |
IgG1 |
nd |
|
R6C2 |
0.25 ± 0.01 |
IgM |
99, 89, 83, 48 |
Table 2.Macrophage invasion and multiplication of tachyzoites after infection by Toxoplasma gondii (RH) pretreated with mAbs
Table 2.
|
Pre-treatment with mAb or seruma)
|
No. of infected cells/100 Mac.b)
|
No. of tachyzoites/cell
|
|
4 h |
18 h |
4 h |
18 h |
|
No serum |
12.1 ± 8.99 |
65.8 ± 11.76 |
2.2 ± 0.73 |
3.8 ± 1.09 |
|
Nl serum |
2.7 ± 1.32*
|
44.4 ± 24.54 |
1.5 ± 0.48 |
2.8 ± 0.71 |
|
Immune serum |
1.0 ± 0.73*
|
5.2 ± 3.09*
|
1.2 ± 0.68*
|
2.1 ± 0.31 |
|
M110 |
3.0 ± 3.51 |
7.8 ± 1.79*
|
1.1 ± 0.60*
|
2.4 ± 0.51 |
|
M556 |
2.7 ± 1.28*
|
18.5 ± 8.07*
|
1.3 ± 0.21*
|
2.6 ± 0.74 |
|
R7A6 |
3.0 ± 1.74 |
18.8 ± 12.62*
|
1.5 ± 0.45 |
2.7 ± 0.92 |
|
M621 |
6.0 ± 4.54 |
28.5 ± 21.57*
|
1.5 ± 0.44 |
3.5 ± 1.87 |
|
Mixed mAbs |
2.5 ± 1.36 |
7.3 ± 2.54*
|
1.8 ± 0.46 |
3.7 ± 1.14 |