Abstract
A 33-year-old Korean man visited a medical clinic with complaints of throat discomfort and pain for one week. The symptoms occurred one day after eating raw brackish water fish, perch. Endoscopy revealed a fluke, about 5 mm in length, attaching to and peristaltically moving on the surface of the mucosa at the arytenoid region of the larynx. Microscopically, the testes were triangular, tandem, and separated by the uterus. The ovary and cirrus pouch were placed apart from median line between testes. Numerous blood cells were observed in the ceca. The worm was identified to be Clinostomum complanatum. This is the second human case of clinostomiasis in Korea.
-
Key words: Clinostomum complanatum, brackish water fish, human case, Korea
INTRODUCTION
Clinostomum complanatum is a digenetic trematode which usually infects fish-eating water birds. If a human consumes raw fish, the fluke is accidentally attached on the surface of mucus membrane of the throat and causes a clinical syndrome called halzoun. Since the first human infection with
C. complanatum was reported in Japan [
1], many cases have been described in Japan and other countries. In Korea,
C. complanatum is also indigenously distributed. According to previous epidemiological studies, 12 species of freshwater fish were recorded as the second intermediate hosts [
2,
3], and 1 species of freshwater snail was reported as the first intermediate host of
C. complanatum [
4]. A human case of
Clinostomum infection was reported in a Korean man who had eaten several kinds of raw freshwater fish he caught [
5]. Here, we describe a second case of human infection with
Clinostomum in the mucous membrane of the larynx, which was diagnosed by endoscopy and extraction of the worm.
CASE REPORT
A Korean man, 33-year-old, came to the outpatient's department of internal medicine, 21C HANA Medical Clinic, Mokpo, Korea. The patient complained of throat discomfort and pain of 1 week duration. Strange sensation in his throat occurred 1 day after eating raw marine fish, a perch.
Vital signs were stable. Laboratory data showed the following values; hemoglobin, 14.5 g/dl, leukocyte count, 6,500/µl (70% neutrophils, 27% lymphocytes, and 2% eosinophils), platelet, 247,000/µl, AST, 21 IU/L, ALT, 21 IU/L, γ-GPT, 26 IU/L, ALP, 136 IU/L, total serum protein, 7.3 g/dl with albumin, 4.5 g/dl. Occult blood was negative and no helminth eggs were found in routine stool examination.
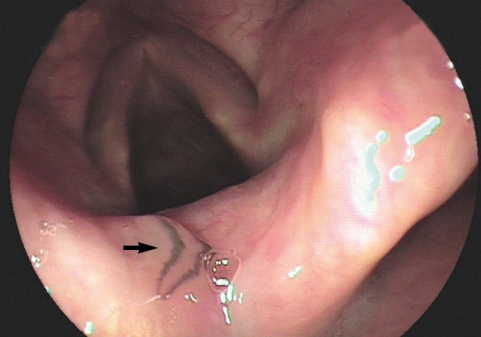
During a drug-induced sleep endoscopy for the upper airway and esophagus evaluation, a motile fluke was found attached to the surface of the arytenoid region (
Fig. 1). The worm moving its posterior portion of the body peristaltically was immediately removed from the mucosa. It was partially torn at the lateral part of the middle one third when it was grasped by endoscopic forceps (
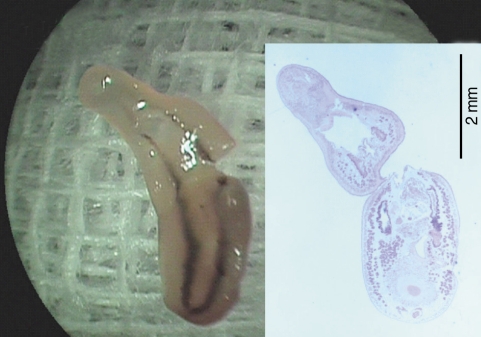
Fig. 2). After removal of the worm, petechial hemorrhage was observed on the affected mucosa. The pain and other symptoms were gradually relieved. In unfixed condition, the fluke was 5.25 mm in length, 1.81 mm in maximum width at the posterior one third of the body. The oral sucker was located near the frontal end of the body. The ventral sucker was large and situated at the middle of the anterior one third of the body. The intestine of the worm was heavily dark due to ingested materials at its middle and posterior one third portion.
Longitudinal sections of the worm were prepared and stained with hematoxylin and eosin (
Figs. 2,
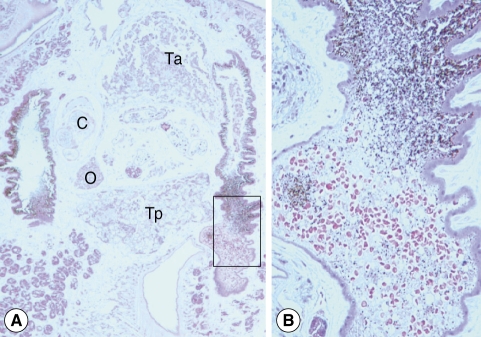
3). Morphological observation was performed by light microscopy, and measurements of internal organs were done with a computer-aided image analysis system (analySIS, SIS GmbH, Duisburg, Germany). Testes were roughly triangular in shape (
Fig. 3A). They were tandem and separated by the posterior part of the uterus. The anterior testis was more triangular and situated in the posterior part of the middle one-third body, measuring 420 × 560 µm. The posterior testis, crescentic and anteriorly concave, was located in the anterior part of the posterior one third, 340 × 620 µm. The ovary, triangular and 150 × 150 µm, was placed apart from the median line between the cirrus pouch and posterior testis. The cirrus pouch, oval and 380 × 230 µm, was situated anterolaterally to the ovary. Some part of the uterus, egg containing, was located between the anterior and posterior testes. Vitellaria, follicular, were distributed from the post-acetabular level to the posterior end of the body along the lateral margins. Intestinal ceca bifurcated and ran posteriorly. They contained a lot of host red blood cells and pigments stained in brown and black color (
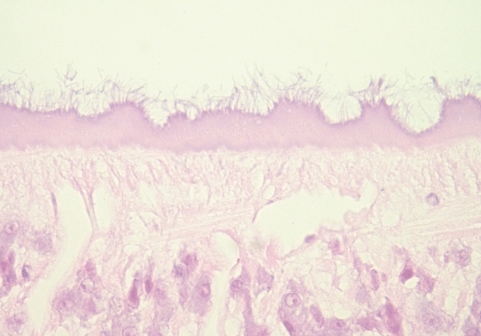
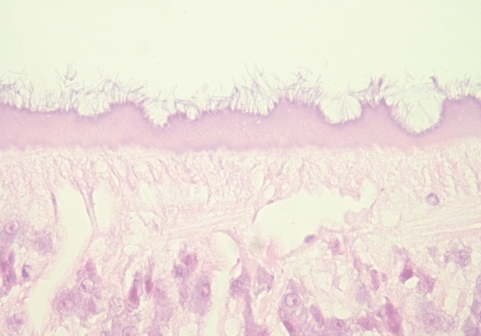
Fig. 3B). The undigested cells were abundant mainly in the posterior portion of the ceca. The pigments were found in clusters adhering to the luminal surface of gastrodermis as well as lying free and mixed with remnants of undigested red blood cells in the lumen. No tegumental spine was noticed. The outer surface of the tegument, however, was occupied by numerous filamentous materials (
Fig. 4). Their size and distribution was variable along the body. From these findings, the present worm was identified as
Clinostomum complanatum.
DISCUSSION
Clinostomum species are common fish parasites throughout the world. The final hosts of this fluke are generally piscivorous birds, including herons and egrets [
6]. The metacercariae embedded in the tissues of fish are freed in the host stomach and migrates up to the esophagus, and then attach to the throat or mouth cavity. Human infections are known to be resulting from eating raw freshwater fish. In Korea, a previously reported human case occurred in an otherwise healthy 56-year-old man from Taegu in 1994 [
5]. The present case, therefore, constitutes the second documented case of clinostomiasis in Korea.
Our patient had a recent history of eating raw fish during his 10-day trip to Mokpo. He consumed perch,
Lateolabrax japonicus, 1 day before the onset of symptoms, but denied ever eating freshwater fish raw. In view of the onset of his symptom, the brackish water fish, perch, was probably responsible as the source of infection. Therefore, this is the first case of
C. complanatum infection by eating brackish water fish in Korea. Some brackish water fish are considered to be natural intermediate hosts for various
Clinostomum species in many countries. The presence of
C. complanatum was registered in Pacific fat sleeper,
Dormitator latifrons which lives in brackish waters and is distributed from California to Ecuador living in the coastal slopes of the Pacific [
7]. In Japan, it was reported that perch,
L. japonicus, serve as the second intermediate host of
C. complanatum [
8]. Korea and Japan share the sea and fish, and this fish is commonly seen and eaten raw in Korea. A human case, the infection of which was suspected to be caused by marine fish, also occurred in a 20-year-old Japanese woman residing in Yamaguchi City [
9]. She ate raw marine fish and cuttlefish caught at the seashore nearby her house. Therefore, the possible role of brackish water fish as the source of human infection should be further investigated in Korea.
In case of accidental human infections,
Clinostomum attaches to the mucous membrane of the throat. It usually causes acute pharyngitis or laryngitis; most cases are not serious and can even be self-limiting. If symptoms called halzoun occur after eating uncooked fish, the primary therapy is endoscopic removal of the parasitic worm. Capture of
Clinostonum, however, is often difficult because of its rapid movement and firm adhesion to the mucosa. Some otolaryngologist tried local anesthetic spray on the parasite. They insisted that administration of 8% lidocaine solution was very effective not only to paralyze the worm movement but also to eliminate its suction to mucosa, allowing it to be easily removed [
10]. In the present case, local anesthetic spray was not considered because the worm movement was not so fast. The parasite, however, was attached to the arytenoid mucosa so firmly that it was partially broken when it was removed. In general, retrieval of intact parasite is extremely important in parasitological identification and differential diagnosis. Therefore, we agree to the recommendation to use local anesthetic spray to
Clinostomum to keep the parasite body intact and allow easy removal.
The nutrition of
Clinostomum has been studied, but, in general, it is restricted to glucose uptake across the external surface. Consequently, only a limited amount of information is available regarding the oral uptake. According to the histological study, the oral sucker of
Clinostomum engulfs the mucous layer of the throat or mouth of bird hosts, and the intense liquefactive necrosis of engulfed tissue occurs [
11]. In the present case, petechial hemorrhage was observed endoscopically after the removal of the parasite. Histological observation on the ceca of the worm revealed numerous blood cells and fine granules of the brown-black pigment in the lumen in contact with the gastrodermis. The pigment looked like a degradation product of hemoglobin by digestive process. Thus, it is suggested that
C. complanatum could feed on blood extracted from the mucosa and cause mucosal hemorrhage due to attachment injury.
Comparing the measurements of
C. complanatum, there were differences in worm size among reported human cases according to the retrieval period. The worm retrieved from a human was 4.5 mm long and 1.8 mm wide at day 2 after infection [
10]. It grows to be 4.74 mm long and 1.05 mm wide at days 3-4 [
5], and 5.17 mm long and 2.00 mm wide at day 7 [
9]. At day 30 after infection, it grows up to 7.00 mm long and 2.43 mm wide [
12]. This means that
C. complanatum grows well in the throat of humans, an accidental host. The size of the present worm was similar to that removed at day 7 after infection. In comparison with the surface morphology, the tegument is devoid of surface spines in the present case. This is corresponded with the first Korean case reported by Chung et al. [
5]. However, the sectioned worm displayed a distinctively hirsute appearance on its outer surface of the tegument under light microscopy. Presence of microorganisms on the external surface of trematodes has been reported infrequently.
Clinostomum species also have various ectocommensal microorganisms, including bacteria and yeast on the outer surface of the tegument [
13,
14]. Thus, further studies using an electron microscope may be needed to elucidate the nature of filamentous materials of the tegument.
References
- 1. Yamashita J. Clinostomum complanatum, a trematode parasite new to man. Annot Zool Japan 1938;17:563-566.
- 2. Chung DI, Kong HH, Moon CH. Demonstration of the second intermediate hosts of Clinostomum complanatum in Korea. Korean J Parasitol 1995;33:305-312.
- 3. Rim HJ, Kim KH, Joo KH, Kim SJ, Eom KS, Chung MS. The infestation states and changing patterns of human infecting metacercariae in freshwater fish in Kyongsang-do and Kyonggi-do, Korea. Korean J Parasitol 1996;34:95-105.
- 4. Chung DI, Kong HH, Joo CY. Radix auricularia coreana: Natural snail host of Clinostomum complanatum in Korea. Korean J Parasitol 1998;36:1-6.
- 5. Chung DI, Moon CH, Kong HH, Choi DW, Lim DK. The first human case of Clinostomum complanatum (Trematoda: Clinostomidae) infection in Korea. Korean J Parasitol 1995;33:219-223.
- 6. Aohagi Y, Shibahara T, Machida N, Yamaga Y, Kagota K, Hayashi T. Natural infections of Clinostomum complanatum (Trematoda: Clinostomatidae) in wild herons and egrets, Tottori Prefecture, Japan. J Wildl Dis 1992;28:470-471.
- 7. Violante-Gonzalez J, Aguirre-Macedo ML, Vidal-Martinez VM. Temporal variation in the helminth parasite communities of the Pacific fat sleeper, Dormitator latifrons, from Tres Palos Lagoon, Guerrero, Mexico. J Parasitol 2008;94:326-334.
- 8. Aohagi Y, Shibahara T, Kagota K. Metacercariae of Clinostomum complanatum found from new fish hosts, Lateolabrax japonicus and Leuciscus hakonensis. Jpn J Parasitol 1995;44:340-342.
- 9. Kifune T, Ogata M, Miyahara M. The first case of human infection with Clinostomum (Trematoda: Clinostomidae) in Yamaguchi Prefecture, Japan. Med Bull Fukuoka Univ 2000;27:101-105.
- 10. Kitagawa N, Oda M, Totoki T, Washizaki S, Oda M, Kifune T. Lidocaine spray used to capture a live Clinostomum parasite causing human laryngitis. Am J Otolaryngol 2003;24:341-343.
- 11. Dias ML, Eiras JC, Machado MH, Souza GT, Pavanelli GC. The attachment of Clinostomum sp. (Digenea, Clinostomidae) to the oesophagus of the bird Ardea cocoi (Aves, Ardeidae). Parasite 2003;10:185-187.
- 12. Yoshimura K, Ishigooka S, Satoh I, Kamegai S. Clinostomum complanatum from the pharynx of a woman in Akita, Japan: a case report. Jpn J Parasitol 1991;40:99-101.
- 13. Uglem GL, Larson OR, Aho JM, Lee KJ. Fine structure and sugar transport functions of the tegument in Clinostomum marginatum (Digenea: Clinostomatidae): environmental effects on the adult phenotype. J Parasitol 1991;77:658-662.
- 14. Aho JM, Uglem GL, Moore JP, Larson OR. Bacteria associated with tegument of Clinostomum marginatum (Digenea). J Parasitol 1991;77:784-786.
Fig. 1Photographs of the whole worm of Clinostomum complanatum. Endoscopic image showing the parasitic fluke (arrow) attached to the arytenoid region of the larynx.
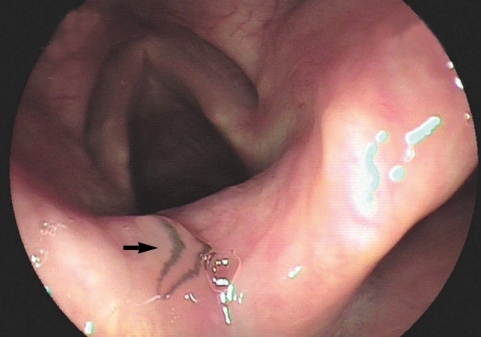
Fig. 2Photographs of the whole worm of Clinostomum complanatum. Gross image of the fluke partially torn during the removal procedure, 5.25 mm in length and 1.81 mm in width. (Inset) A longitudinal section of the fluke showing characteristic internal organs, H-E stain, × 13.
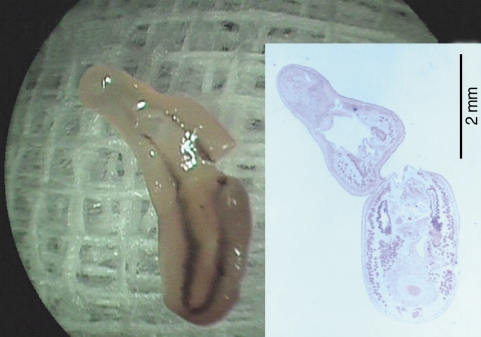
Fig. 3Histological sections of C. complanatum obtained from the patient. (A) Typical distribution of the main reproductive organs. C, cirrus pouch; O, ovary; Ta, anterior testis; Tp, posterior testis. H-E stain, × 40. (B) An enlarged view of the cecum showing ingested blood cells and numerous fine granules of the brown-black pigment. H-E stain, × 200.
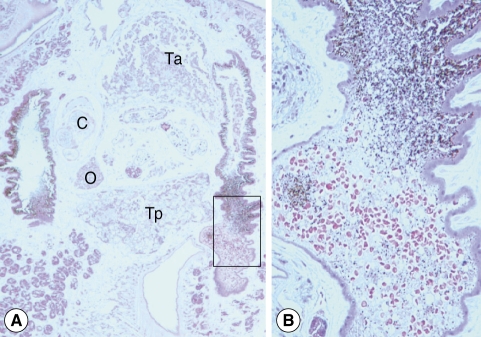
Fig. 4Histological sections of C. complanatum obtained from the patient. Detail of the tegument showing the numerous filamentous materials at the outer surface. H-E stain, × 400.