Abstract
This article analyzed the infection characteristics of metacercariae of Metagonimus spp. (MsMc) in fish from 9 major water systems in Korea. A total of 19,568 fish in 87 species were examined over a period of 10 years (2011–2020). MsMc were detected in fish from all 44 survey areas in 9 water systems. Most of the surveyed sites showed very low and low infection levels (66.7%), while 33.3% of the areas, such as Tamjin-gang and Seomjin-gang, revealed moderate and high infection levels. High endemicity depends on the abundance of susceptible fish species, especially sweet smelt (Plecoglosus altivelis). The susceptibility index (SI) with MsMc in index fish, Zacco spp., was very low and low levels in 62.0%, moderate in 28.0%, and high in 10.0% regions. The SI was highest in the following order: Yeongam-cheon (283.8), Hoeng-cheon (192.3), Togyo-jeosuji (131.2), Deokcheon-gang (119.1), and Joyang-gang (106.3). The recent infection status of MsMc in P. altivelis was analyzed by the survey localities. In addition, except for P. altivelis, 9 fish species were highly infected with MsMc in some survey areas, including Zacco platypus, Z. koreanus, Z. temminckii, Opsariichthys uncirostris, Rhynchocypris oxycephalus, Carassius auratus, Acheilognathus rhombeus, Onchorhynchus masou, and Tribolodon hakonensis. In Korea, 74 fish species (15 families) are collectively listed as second intermediate hosts of Metagonimus spp. This review provides several novel characteristics of MsMc infection and clarifies the fish species of second intermediate host of Metagonimus spp. in this country.
-
Key words: Metagonimus spp., metacercaria, endemicity, susceptibility index, second intermediate fish host
Background and Purpose
Helminthiases had been prevalent nationwide in Korea until the 1970s. The soil-transmitted helminthiases, such as ascariasis, trichuriasis, and hookworm infections were the main public health problems to be addressed at that time. Their prevalence has been drastically decreased by systematic nationwide control programs [
1]. These dieases are no longer a health problem today, while the food-borne helminthiases, including clonorchiasis and metagonimiasis, continues to persist in riverside areas of Korea. The oriental liver fluke,
Clonorchis sinensis, has been considered the most important helminth, and clonorchiasis has been a major endemic disease to be controlled in a project of the Division of Vectors and Parasitic diseases of Korea Disease Control and Prevention Agency (Korea DCPA). Clonorchiasis eradication project has been successfully conducted by Korea DCPA. However, metagonimiasis caused by infection with
Metagonimus spp., is more or less neglected, even though it is another health problems among food-borne helminthiases in Korea [
2–
7]. The long history of metagonimiasis is currently being confirmed through molecular researches emplying archaeological specimens from Joseon Dynasty mummies [
8,
9].
Fluke members in the genus
Metagonimus Katsurada, 1912 (Digenea: Heterophyidae) included more than 7 nominal species; i.e.,
M. yokogawai Katsurada, 1912,
M. takahashii Suzuki, 1930,
M. minutus Katsuta, 1932,
M. katsuradai Izumi, 1935,
M. otsurui Saito and Shimizu, 1968,
M. miyatai Saito et al., 1997, and
M. hakubensis Shimazu, 1999 [
10,
11]. Recently, 2 species,
M. suifunensis Shumenko et al., 2017 and
M. pusillus Tatonova et al., 2018, were additionally reported in Russia [
12,
13]. Among these 9
Metagonimus species, 3 ones, i.e.,
M. yokogawai,
M. takahashii, and
M. miyatai, are distributed in this country. These flukes casue an important food-borne zoonotic disease in Korea [
2,
10,
11]. Especially, infections by
M. yokogawai are mainly prevalent in the riverside areas of eastern and southern coast of Korean peninsula [
14–
20]. Human cases by
M. takahashii were first reported in inhabitants of Eumseong-gun (gun=county), Chungcheongbuk-do (do=Province), along the upper reaches of the Namhan-gang (gang means river) [
21].
M. miyatai infection was endemic among peoples residing around lakes and along the rivers and/or streams in inland of Korea [
22–
24]. These
Metagonimus species cause severe gastrointestinal troubles and chronic diarrhea when heavily infected [
10,
11].
Since metacercaria of
Metagonimus yokogawai (MyMc) was first discovered in Korea from a crusian carp (
Carassius auratus), many fish species have been reported as the second intermediate hosts of
Metagonimus spp., which is the source of infection [
25,
26]. The sweet smelt,
Plecoglossus altivelis, and the crusian carp,
C. auratus, were reported to be intermediate hosts of MyMc and
M. takahashii metacercariae (MtMc), respectively, from Miryang-gang in Gyeongsangnam-do [
27,
28]. Kang et al. [
29] detected MyMc in sweet smelt from Jeju-do, and Choi et al. [
30] found MyMc in the sea rundace,
Tribolodon hakonensis, from Hyeongsan-gang in Gyeongsangbuk-do. Thereafter, many workers reported the infections of
Metagonimus spp. metacercariae (MsMc) in variety of fish species from various survey regions [
31–
48]. Rim et al. [
47] performed a study to clarify the host (fish) specificity of MsMc with morphologies of adults, which were recovered from hamsters experimentally infected with MsMc from sweet smelt, crusian carp, and chubs (
Zacco platypus, Z. temminckii, and
Opsarichthys uncirostris amurensis). In Korea, 3 species of fish, i.e., sweet smelt, sea rundace, and Japanese seabass (
Lateolabrax japonicus) were clearly known as second intermediate hosts of
M. yokogawai [
15–
18]. The crusian carp, common carp (
Cyprinus carpio), sea rundace, and Japanese seabass were reported as second intermediate hosts of
M. takahashii [
15,
18,
19]. Many species of fish including the sea rundace and chubs were listed as second intermediate hosts of
M. miyatai in Korea [
15,
18,
20].
Infecton status of MsMc was frequently examined in sweet smelts from the specific regions in Korea [
14–
18,
27,
29,
33–
35,
37,
38,
41,
43,
48]. A previous study surveyed the infection status of zoonotic trematode metacercariae (ZTM), including
Metagonimus spp., in freshwater fish from Gangwon-do [
49]. Sohn et al. [
50] investigated the infection status of digenetic trematode metacercariae (DTM) in freshwater fish from the water systems of Hantan-gang and Imjin-gang located in relatively northern regions of Korea. The infection status with MsMc in fishes from Seomjin-gang and Tamjin-gang was also recorded [
51]. The riverside areas of Seomjin-gang and Tamjin-gang has been known as the endemic areas of heterophyid flukes including
M. yokogawai [
52–
56]. Sohn et al. [
57,
58] reported the infection status of ZTM in fish from Geum-gang and of DTM in fish from coastal lakes in Gangwon-do. Sohn and Na [
59] described the infection status of DTM in freshwater fishes from 2 visiting sites, Junam-jeosuji (jeosuji means reservoir) and Woopo-neup (neup means swamp), of migratory birds in Gyeongsangnam-do. Recent study [
60] investigated the infection status of ZTM in freshwater fishes from Soyang-cheon in Wanju-gun, Jeollabuk-do during 2 survey periods, the former (2013–2015) and the latter periods (2018–2019). Sohn et al. [
61,
62] intensively investigated the infection status of ZTM in fish from the irrigation canal of Togyo-jeosuji in Cheorwon-gun, Gangwon-do for 3 years (2018–2020), and from Deokcheon-gang in Sancheong-gun, Gyeongsangnam-do.
Individual studies on the metacercarial infections in fish hosts were continuously performed, but the prevalence and intensity of MsMc infections in fish intermediate hosts have not been systematically and extensively analyzed in Korea. This article reviewed the data from published and unpublished results obtained from studies on the detection of MsMc by survey localities and by fish species in our laboratory during the past 10 years (2011–2020). The status of MsMc infection was analyzed on a total of 19,568 fishes in 87 species from 9 main water systems in Korea, which included Hantan-gang and Imjin-gang, Han-gang, Geum-gang, Mangyeong-gang, Yeongsan-gang, Tamjin-gang, Seomjin-gang, Nakdong-gang, and streams in the east coastal areas.
Data collection and analysis
All collected fishes were kept on ice and transported to the laboratory of the Department of Parasitology and Tropical Medicine, Gyeongsang National University College of Medicine, Jinju, Korea. After identifying the species, each fish was finely ground in a mortar with pestle. The ground fish meat was mixed with artificial gastric juice, and incubated at 36°C for about 2 h. The digested material was filtered through a mesh (pore size 1×1 mm) and washed with 0.85% physiological saline until the supernatant became clear. The sediment was carefully examined under a stereomicroscope.
Metagonimus spp. metacercariae (MsMc:
Fig. 1) were separately collected according to previously described method [
26]. MsMc were counted to determine the infection rate (No. of fish with MsMc/No. of fish examined×100) and intensity (No. of MsMc detected/No. of fish infected) by fish species. The endemicity was calculated by the formula, positive rate (No. of positive fish species/No. of fish spp. examined) of fish spp. ×positive rate (No. of positive fish/No. of fish examined) in positive fish spp. (PFS)×mean No. of MsMc detected, and it was divided into 4 groups, i.e., very low (below 3.0), low (3.0–25.0), moderate (25.1–75.0), and high (over 75.1), by the endemic index. The susceptibility index (SI) of MsMc in index fish,
Zacco spp., was calculated by the formula, prevalence/100×mean metacercarial intensity per fish infected (PFI), and it was also divided into 4 groups, i.e., very low (below 5.0), low (5.01–30.0), moderate (30.1–100.0), and high (over 100.1), by survey regions.
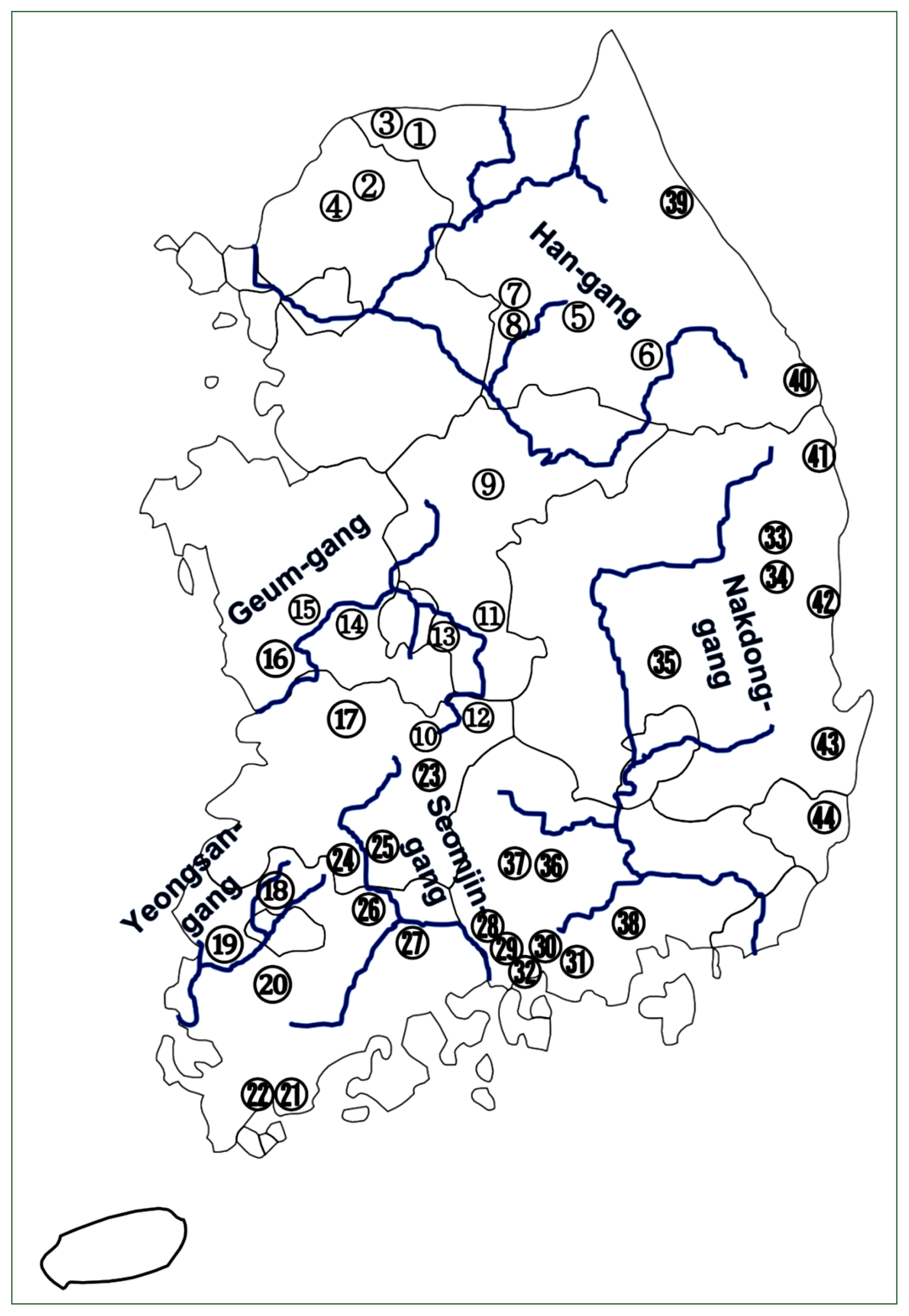
The overall number of fishes examined by the survey localities is presented in
Table 1 and each survey locality is marked in
Fig. 2.
A total of 540 fishes (32 species) were examined in ① Hantan-gang (Latitude: 38.23047; Longitude: 127.2179) in Cheorwon-gun, Gangwon-do (2012, 2013, 2019). In ② Hantan-gang (37.94375; 127.07142) in Yeoncheon-gun, Gyeonggi-do (2013), a total of 195 fish in 13 spp. were examined. A total of 568 fishes in 19 spp. were examined in ③ Togyo-jeosuji (38.27082; 127.28949), Cheorwon-gun, Gangwon-do (2018–2020). In ④ water systems of Imjin-gang (2013), a total of 283 fish in 15 spp. were examined. Fish species and the number of fish examined were designated by survey localities in
Supplementary Table S1 in detail.
A total of 228 fishes (15 spp.) were examined in ⑤ Pyeongchang-gang (37.32968; 128.37765) in Pyeongchang-gun, Gangwon-do (2013). In ⑥ Joyang-gang (37.44292; 128.66256) in Jeongseon-gun, Gangwon-do (2012), a total of 196 fish in 15 spp. were examined. A total of 184 fish in 20 species were examined in ⑦ Seom-gang (37.50058; 127.99337) in Hoengseong-gun, Gangwon-do (2011). In ⑧ Seom-gang (37.42690; 127.89634) in Wonju-si, Gangwon-do (2018–2020), a total of 707 fish in 30 spp. were examined. A total of 99 fish in 12 species were examined in ⑨ Dal-cheon in Goesan-gun, Chungcheongbuk-do (2011).
Supplementary Table S2 shows the fish species and the number of fish examined according to the survey localities.
A total of 208 fish (19 spp.) were examined in ⑩ Juja-cheon (35.98023; 127.39388), the upper stream of Geum-gang, in Jinan-gun, Jeollabuk-do (2012). In ⑪ Chogang-cheon in Yeongdong-gun, Chungcheongbuk-do (2011), 132 fish in 15 spp. were examined. One hundred twenty-three fish (14 spp.) were examined in ⑫ Geum-gang (35.97529; 127.55662) in Muju-gun, Jeollabuk-do (2012). A total of 365 fish (25 spp.) were examined in ⑬ Geum-gang (36.11427; 127.58775) in Geumsan-gun, Chungcheongnam-do (2011, 2013, 2015). In ⑭ Yugu-cheon (36.53727; 126.94847) in Gongju-si, Chungcheongnam-do (2013, 2015), 311 fish (14 spp.) were examined. A total of 89 fish (13 spp.) were examined in ⑮ Ji-cheon (36.38958; 126.85174) in Cheongryang-gun, Chungcheongnam-do (2013). In ⑯ Nonsan-cheon (36.19906; 127.06790) in Nonsan-si, Chungcheongnam-do (2013), 44 fish (11 spp.) were examined. Fish species and the number of fish examined are shown by survey localities (
Supplementary Table S3).
A total of 607 fish in 18 spp. were examined in ⑰-1 Soyang-cheon (36.11427; 127.58775) in Wanju-gun, Jeollabuk-do (2012–2015). In same survey locality, ⑰-2 Soyang-cheon (2018, 2019), a total of 465 fish in 25 spp. were examined. Fish species and the number of fish examined are demonstrated in
Supplementary Table S4.
In ⑱ Hwangryong-gang (35.29296; 126.77087) in Jangseong-gun, Jeollanam-do (2011), 107 fish in 14 species were examined. A total of 140 fish in 14 species were examined in ⑲ Jiseok-cheon (35.04768; 126.80448) in Hwasun-gun, Jeollanam-do, (2011, 2013). In ⑳ Yeongam-cheon (35.04086; 126.65664) in Yeongam-gun, Jeollanam-do (2013), 45 fish (8 spp.) were examined. Fish species and the number of fish examined were shown by survey localities in
Supplementary Table S5.
A total of 712 fish (21 spp.) were examined in ㉑-1 Tamjin-gang (34.42572; 126.54322) in Jangheung-gun, Jeollanam-do (2014–2016). In ㉑-2 Tamjin-gang, Jangheung-gun, Jeollanam-do (2017–2019), 972 fish in 25 spp. were examined. A total of 517 fish (17 spp.) were examined in ㉒ Tamjin-gang (34.38053; 126.48514) in Gangjin-gun, Jeollanam-do (2014, 2017). Fish species and the number of fish examined were designated in
Supplementary Table S6 in detail.
In ㉓ Osu-cheon (35.52768; 127.32885) in Imsil-gun, Jeollabuk-do (2011–2013), 341 fish (15 spp.) were examined. A total of 310 fish in 29 spp. were examined in ㉔-1 Seomjin-gang (35.43854; 127.24047) in Sunchang-gun, Jeollabuk-do (2014, 2015). In ㉔-2 Seomjin-gang in Sunchang-gun, Jeollabuk-do (2018–2020), 676 fish (32 spp.) were examined. A total of 420 fish in 25 spp. were examined in ㉕ Songdae-cheon (35.91616; 127.15413) in Namwon-si, Jeollabuk-do (2012, 2013, 2020). Fish species and the number of fish examined were shown by survey localities (
Supplementary Table S7).
In ㉖-1 Seomjin-gang (35.14903; 127.32589) in Gokseong-gun, Jeollanam-do (2015, 2016), 289 fish in 15 spp. were examined. A total of 631 fish in 22 spp. were examined in ㉖-2 Seomjin-gang in Gokseong-gun, Jeollanam-do (2018–2020). In ㉗ Seomjin-gang (35.14340; 127.31661) in Gurye-gun, Jeollanam-do (2014, 2020), 356 fish (28 spp.) were examined. Fish species and the number of fish examined are presented by survey localities (
Supplementary Table S8).
A total of 202 fish in 15 spp. were examined in ㉘ Hwagye-cheon (35.02828; 127.81974) in Hadong-gun, Gyeongsangnam-do (2020). In ㉙ Akyang-cheon (35.16218; 127.71133) in Hadong-gun, Gyeongsangnam-do (2018), 179 fish in 12 spp. was examined. A total of 328 fish in 14 spp. were examined in ㉚ Namsan-cheon (35.09540; 127.79806) in Hadong-gun, Gyeongsangnam-do (2015, 2016, 2019). In ㉛ Hoeng-cheon (35.10716; 127.80779) in Hadong-gun, Gyeongsangnam-do (2014, 2016, 2019), 318 fish in 13 spp. were examined. A total of 196 fish in 23 spp. were examined in ㉜ Jugyo-cheon (35.02828; 127.81974) in Hadong-gun, Gyeongsangnam-do (2011, 2018). Fish species and the number of fish examined were shown by survey localities in
Supplementary Table S9.
A total of 161 fish (12 spp.) were examined in ㉝ Banbyun-cheon (36.59338; 129.06975) in Yeongyang-gun, Gyeongsangbuk-do (2015). In ㉞ Yongjeon-cheon (36.40716; 129.36594) in Cheongsong-gun, Gyeongsangbuk-do (2019, 2020), 804 fish in 20 spp. was examined. Fish species and the number of fish examined are shown by survey localities in
Supplementary Table S10.
We examined 550 fish in 26 spp. in Wi-cheon (㉟-1, 36.18863; 128.64873) in Gunwi-gun, Gyeongsangbuk-do (2011, 2013, 2014). In Wi-cheon (2015–2017), 723 fish in 33 spp. were examined (㉟-2). A total of 782 fish in 28 spp. were examined in Wi-cheon (㉟-3) in 2018–2020. Fish species and the number of fish examined by survey period are presented in
Supplementary Table S11.
A total of 1,357 fish (23 spp.) were examined in Yang-cheon (㊱-1, 35.36021; 128.05820) in Sancheong-gun, Gyeongsangnam-do (2011–2014). In Yang-cheon (㊱-2, 2015–2017), 844 fish in 23 spp. were examined. A total of 871 fish 19 spp. were examined in ㊲ Deokcheon-gang (35.24643; 127.89224) in Sancheong-gun, Gyeongsangnam-do (2018–2020). In ㊳ Jisu-cheon (35.33582; 128.32520) and Haman-cheon (35.20562; 128.44302) in Jinju-si and Haman-gun, Gyeongsangnam-do (2015), 167 fish in 13 spp. were examined. Fish species and the number of fish examined by survey localities are shown in
Supplementary Tables S12 and
S13.
A total of 140 fish (13 spp.) were examined in ㊴ Namdae-cheon (38.07302; 128.59303) in Yangyang-gun, Gangwon-do (2015). In ㊵ Osip-cheon (37.42217; 129.11746) in Samcheok-si, Gangwon-do (2015), 143 fish in 15 spp. were examined. A total of 140 fish (13 spp.) were examined in ㊶ Wangpi-cheon (36.96583; 129.39499) in Uljin-gun, Gyeongsangbuk-do (2015). In ㊷ Osipcheon (36.40716; 129.36594) in Yeongdeok-gun, Gyeongsangbuk-do (2015, 2018), 283 fish in 17 spp. were examined. A total of 111 fish in 12 spp. were examined in ㊸ Gigye-cheon (36.03105; 129.24680) in Gyeongju-si, Gyeongsangbuk-do (2015). In ㊹ Cheokgwa-cheon (35.59894; 129.27461) and Taehwa-gang (35.58515; 129.22520) in Ulsan Metropilitan City (2015), 310 fish (17 spp.) were examined. Fish species and the number of fish examined by survey localities are shown in
Supplementary Table S14.
Infection status of MsMc by fish species and survey regions
Hantan-gang and Imjin-gang
The prevalence of MsMc was 55.3%, 33.9%, 74.1%, and 57.4% in positive fish species (PFS) from ① Hantan-gang (Cheorwon), ② Hantan-gang (Yeoncheon), ③ Togyo-jeosuji, and ④ Imjin-gang (
Table 2). The infection intensities were 35.1, 8.6, 62.0, and 9.6 per fish infected (PFI), respectively.
Supplementary Table S1 shows the infection status by the fish species and survey locality.
In the early 1990s, 3.4% egg-positive rate (prevalence) of
Metagonimus spp. from 465 fecal samples of residents in riverside areas of Hantan-gang in Cheorwon-gun, Gangwon-do was reported [
45]. They also reported 33.3% prevalence of MsMc in 7 PFS from this region. Cho et al. [
49] reported 64.9% and 95.6% prevalence of MsMc and 98.8 and 24.0 MsMc intensity of infection in fishes from Hwa-gang (upper reaches of Hantan-gang) and Hantan-gang in Cheorwon-gun. We detected MsMc in 312 (61.1%) out of 511 fishes (in 19 PFS) from the water systems of Hantan-gang, and in 113 (59.0%) out of 191 fish (in 8 PFS) from the water systems of Imjin-gang [
50]. The intensities of infection with MsMc were 47.5 and 9.6 PFI in each survey regions. In case of index fish,
Zacco spp., the prevalence of MsMc were 74.0% and 72.1%, and their infection intensities were 43.9 and 18.6 PFI. Therefore, susceptibility indices (SI) were 32.49 and 13.41 in 2 survey regions of Hantan-gang and Imjin-gang. These SI values were very similar with those (30.09 and 13.19) of this review.
Present study shows that the endemicities with MsMc were 36.25 in ③ Togyo-jeosuji, 14.48 in ① Hantan-gang (Cheorwon), 2.90 in ④ Imjin-gang and 2.02 in ② Hantan-gang (Yeoncheon) respectively. SI of MsMc in the index fish,
Zacco spp., were 131.24 in ③ Togyo-jeosuji, 30.09 in ① Hantan-gang (Cheorwon), 13.19 in ④ Imjin-gang and 3.87 in ② Hantan-gang (Yeoncheon) respectively. In a previous study, SI were 14.23 and 22.61 in
Zacco spp. from Hwa-gang and Hantan-gang in Cheorwon-gun [
49]. Although this survey region (water systems of Hantan-gang and Imjin-gang) is in the northern part of Korea, MsMc is widespread in the PFS and is highly endemic in index fish from Togyo-jeosuji.
The prevalence of MsMc was 66.7%, 70.9%, 74.2%, 44.3%, and 62.9% in PFS from ⑤ Pyeongchang-gang, ⑥ Joyang-gang, ⑦ Seom-gang (Hoengseong), ⑧ Seom-gang (Wonju), and ⑨ Dal-cheon (
Table 2). The infection intensities were 10.0, 60.1, 32.3, 27.4, and 79.4 PFI respectively.
Supplementary Table S2 shows the infection status by the fish species and survey locality.
Some epidemiological studies on metagonimiasis have been conducted in the Han-gang system [
21,
42,
44,
46]. The prevalence of metagonimiasis in 529 fecal samples of inhabitants in the riverside areas of Hongcheon-gang in Hongcheon-gun, Gangwon-do were 7.4% and the infection status with MsMc from 44 pale chubs,
Z. platypus, were 68.2% MsMc positive in PFS with average infection intensity of 26 MsMc [
42]. Chai et al. [
21] surveyed the prevalence of metagonimiasis with 231 fecal samples of inhabitants along the upper reaches of Namhan-gang in Umseong-gun, Chungcheongbuk-do and Yeongwol-gun, Gangwon-do (22.5% egg positivity) and 97.6% of the infection status with MsMc from 5 fish species including 42
Z. platypus. Ahn [
44] reported 7.8% prevalence of metagonimiasis from 1,067 inhabitants residing in the riverside areas of Gangwon-do. He also examined for infections of MsMc in fish from 6 rivers, i.e., Seom-gang, Jucheon-gang, Pyeongchang-gang, Hongcheon-gang, Dong-gang, and Osip-cheon, in Gangwon-do. MsMc prevalence in
Z. platypus was 75.7% (Seom-gang: 112/148), 77.1% (Jucheon-gang: 37/48), 87.5% (Pyeongchang-gang: 28/32), 63.2% (Hongcheon-gang: 12/19), and 81.5% (Dong-gang: 22/27) respectively. MsMC intensities of infection in
Z. platypus were 165 (Seom-gang:
n=25), 56 (Jucheon-gang:
n=20), 35 (Pyeongchang-gang:
n=12), and 68 (Hongcheon-gang:
n=12) respectively. Yu et al. [
46] reported 20.9% egg-positive rate of metagonimiasis from 67 fecal samples of inhabitants along Dal-cheon in Cheongwon-gun, Chungcheongbuk-do. They also detected MsMc in 13 fish species from this region.
The endemicities with MsMc were 5.4 in ⑤ Pyeongchang-gang, 9.6 in ⑦ Seom-gang (Hoengseong), 8.8 in ⑧ Seom-gang (Wonju), and 29 in ⑨ Dal-cheon in this review. The endemicities with MsMc were not able to calculate so the fish species and number of fish examined were not variety and enough in previous studies [
44,
46]. However, SI in index fish were 30.63 in Pyeongchang-gang, 124.91 in Seom-gang [
44], and 100.70 in Dal-cheon [
46]. Meanwhile in our study, SI were 10.88 in
Zacco spp. from Pyeongchang-gang, 23.80 from Seom-gang (Hoengseong), 45.98 from Seom-gang (Wonju), and 80.67 from Dal-cheon. These findings from SI in index fish suggest that the endemicity with MsMc is decreased to some extent in fish from the water systems of Han-gang in these days.
The prevalence of MsMc was 43.6%, 46.1%, 25%, 78.5%, 21.5%, 69.2%, and 40.0% in PFS from ⑩ Juja-cheon, ⑪ Chogang-cheon, ⑫ Geum-gang (Muju), ⑬ Geum-gang (Geumsan), ⑭ Yugu-cheon, ⑮ Ji-cheon, and ⑯ Nonsan-cheon (
Table 2). The infection intensities were 4.2, 14.1, 2.4, 37.5, 2.9, 32.7, and 4.2 PFI, respectively. The infection status by the fish species and survey localities are shown in
Supplementary Table S3.
A previous study reported 21.0% prevalence from 790 inhabitants in the river basin of Geum-gang in Chungcheongnam-do. In addition, MsMc were detected in 275 fish (76.8%) among 358 fish examined in 18 PFS including 106
Z. platypus and 6
Z. temminckii [
22]. However, this study did not provide the data on the infection intensity of MsMc since endemicity and SI could not be calculated [
22]. Another study reported 5.5% prevalence of metagonimiasis from 1,081 inhabitants along the Geum-gang in Okcheon-gun, Chungcheongbuk-do [
24]. Kim et al. [
23] detected MsMc in 7 fish spp. from Daecheong-ho (lake) and upper reaches of Geum-gang. In index fish,
Zacco spp., the prevalence with MsMc was 79.4% (85/107) and 100% (26/26) and infection intensities were 36.1 and 103.5 PFI in these 2 sites. Therefore, SI in index fish were to be 28.66 and 103.50 in each region. Sohn et al. [
56] detected MsMc in 432 (51.7%) out of 835 fishes in PFS from 6 survey localities, where the infection intensity averaged 30 PFI. The findings in 6 survey localities were nearly equal to those of same localities in this review. SI in
Zacco spp. was relatively low in water systems of Geum-gang except for that of 2 regions, Chogang-cheon (74.0) and Geum-gang (Geumsan: 43.7) [
56]. Conclusively, the endemicity of
Metagonimus infections has been gradually decreased in humans and fish intermediate hosts in the riverside areas and water systems of Geum-gang.
The prevalence of MsMc was 35.5% (the former period) and 35.9% (the latter period) in PFS from ⑰ Soyang-cheon in Wanju-gun, Jeollabuk-do (
Table 2). The infection intensities were 3.7 and 19.3 PFI each. The infection status by the fish species and survey localities are presented in
Supplementary Table S4.
An epidemiological study was performed to investigate the infection status of DTM in fish from Mangyeong-gang in the early 1980s [
39]. This study detected 43.2% MsMc in 164 fish in 26 spp. (81.3%), as well as 96.4% out of 28
Z. platypus. Their infection intensity was 25 PFI. A recent study observed the infection status of ZTM in freshwater fish from this survey region during 2 periods (2013–2015 and 2018–2019) [
59]. Among 6 species of ZTM (
C. sinensis,
Metagonimus spp.,
Centrocestus armatus,
Echinostoma spp.,
Clinostomum complanatum, and
Metorchis orientalis) detected, the infection status of
Metagonimus spp. is nearly equal to that of present study. The prevalence of MsMc is nearly equal between 2 periods (35.5% and 35.9%), but the infection intensity of latter period (19.3 PFI) is much higher than that of former period (3.5 PFI) in this review. And then SI in index fish was 2.72 in the former period and 25.97 in the latter period. The higher SI in the latter period is due to the high infection intensity with MsMc (90.4 PFI) from 25 (89.3%)
Z. koreanus. A previous study showed that SI in index fish was 24.10 which is similar with that of the latter period in this study [
39]. The MsMc endemicity might be low in fish from Mangyeong-gang.
The prevalence of MsMc was 55.1%, 55.9%, and 90.0% in PFS from ⑱ Hwangryong-gang, ⑲ Jiseok-cheon, and ⑳ Yeongam-cheon (
Table 2). The infection intensities were 5.9, 5.3, and 202.7 PFI, respectively. The infection status by the fish species and survey locality is presented in
Supplementary Table S5.
The infection status of MsMc has not been reported in Yeongsan-gang basin. This study showed that the prevalence and infection intensity of MsMc were very low and similar in fish from Hwangryong-gang and Jiseok-cheon. However, the prevalence (90.0%) and infection intensity (202.7 PFI) were much higher in fish from Yeongam-cheon. Especially, the index fish, 15 Z. platypus and 4 Z. temminckii, were all heavily infected with MsMc and the infection intensity was 283.8 PFI. The SI in index fish from Yeongam-cheon was 283.77, the highest among all 51 surveys.
Tamjin-gang
The prevalence of MsMc was 65.4%, 48.1%, and 66.4% in PFS from ㉑ Tamjin-gang (Jangheung: the former and the latter periods) and ㉒ Tamjin-gang (Gangjin) (
Table 2). The infection intensities were 152.5, 63.1, and 121.1 PFI, respectively. The infection status by the fish species and survey localitis is presented in
Supplementary Table S6.
The riverside areas of Tamjin-gang have been known as the endemic area of intestinal fluke including
Metagonimus spp. and heterophyid flukes [
2,
14,
20,
51–
54,
63]. Chai et al. [
14] reported 26.4% prevalence of metagonimiasis from 606 residents in the riverside areas of Tamjin-gang. Seo et al. treated 14 residents of the Tamjin river basin with praziquantel and MgSO
4, and recovered an average of 21,130 (2,886–63,587)
Metagonimus worms from the patients [
63]. Lee et al. recovered an average of 871 (245–1,219)
Metagonimus worms from 6 inhabitants of the same village 23 years later [
20]. Metagonimiasis endemicity, in terms of worm burden and/or infection intensity, decreased significantly between the 2 survey periods in this area.
Chai et al. [
14] detected 15,688 (144–49,956) MsMc PFI in 20
P. altivelis, from Tamjin-gang. A recent study surveyed the infection status of MsMc in fish from Tamjin-gang in Jeollanam-do [
51]. The prevalence was 56.1% and 66.4% and the infection intensity were 147 and 121 MsMc PFI in fish from the middle reaches in Jangheung-gun and the lower reaches in Gangjin-gun, respectively. SI in the index fish,
Zacco spp., was 38.28 and 32.90 in 2 regions. In case of sweet smelt,
P. altivelis, the SI was 1,313.56 and 841.0 in 2 regions. In this study, the prevalence of MsMc was 65.4%, 48.1%, and 66.4% in PFS from Tamjin-gang (Jangheung: the former and the latter periods and Gangjin). The infection intensities were found to be 152.5, 63.1, and 121.1 MsMc PFI, respectively. SI was 30.0, 65.14, and 32.94 in index fish,
Zacco spp., and that of sweet smelt,
P. altivelis, was 1,257.75, 1,476.0, and 841.0, respectively. These collective data suggest that the endemicity of MsMc has been continuously maintained in fish from Tamjin-gang and much affected with the number of sweet smelt examined.
The prevalence of MsMc was 30.1%, 49.8%, 46.3%, 62.3%, 72.4%, 64.5%, 66.6%, 72.7%, 84.6%, 57.3%, 61.9%, and 73.6% in PFS from ㉓ Osu-cheon, ㉔ Seomjin-gang (Sunchang: the former and the latter periods), ㉕ Songdae-cheon, ㉖ Seomjin-gang (Gokseong: the former and the latter periods), ㉗ Seomjin-gang (Gurye), ㉘ Hwagye-cheon, ㉙ Akyang-cheon, ㉚ Namsan-cheon, ㉚ Hoeng-cheon, and ㉛ Jugyo-cheon, respectively (
Table 2). The infection intensities were 3.7, 86.8, 7.1, 21.8, 43.1, 25.0, 281.5, 800.5, 104.4, 61.8, 127.7, and 9.6 PFI, respectively. The infection status by the fish species and survey locality is presented in
Supplementary Tables S7–
S9.
It has been known that the riverside area of Seomjin-gang, especially Hadong-gun in Gyeongsangnam-do, is an endemic area of metagonimiasis in Korea. Yeo and Seo [
64] reported 47.5% prevalence in 221 inhabitants of Hadong-gun at the early 1970s. Hong and Seo [
33] detected 3,851 MsMc PFI in 10 sweet smelt from Hwagae-myeon, Hadong-gun. Kim et al. [
16] reported 29.1% prevalence in 1,163 inhabitants of Hadong-gun with 2,455 MsMc PFI in 14 sweet smelt from the survey region. Chai et al. [
65] detected 584 MsMc PFI in 15 sea rundace,
T. hakonensis, from Seomjin-gang in Hadong-gun. However, except for 1 study [
51], no epidemiological study on the status of MsMc infection in Seomjin-gang fish has been conducted extensively and systematically. Sohn et al. [
51] investigated the infection status of MsMc in fish from 3 tentative regions, the upper (Osu-cheon in Imsil and Seomjin-gang in Sunchang), middle (Songdae-cheon in Namwon and Seomjin-gang in Gokseong), and lower (Seomjin-gang in Gurye, Namsan-cheon and Hoeng-cheon in Hadong) reaches of Seomjin-gang over 5 years (2012–2016). In this review, I also analyzed the unpublished epidemiological data (2017–2020) on the infection status of MsMc in fish from Seomjin-gang together with previously published data [
51].
The prevalence of MsMc was 36.3%, 49.8%, 64.5%, 43.1%, 78.8%, 58.9%, and 73.3% in PFS from Osu-cheon, Seomjin-gang (Sunchang), Songdae-cheon, Seomjin-gang (Gokseong), Seomjin-gang (Gurye), Namsan-cheon, and Hoeng-cheon [
51] with infection intensities of 4.2, 86.8, 39.9, 43.1, 246.5, 67.5, and 173.6 PFI, respectively. The SI of MsMc from the upper, middle, and lower reaches of Seomjin-gang fish was 25.93, 29.04, and 114.51, repectively. The SI in index fish,
Zacco spp., was 2.52, 18.90, 45.30, 18.35, 98.27, 75.39, and 244.44 in same regions [
51]. The MsMc SI of the upper, middle, and lower reaches of Seomjin-gang
Zacco spp. was 9.93, 32.37, and 134.95. In this study, the prevalence of MsMc was 47.9%, 66.9%, and 67.2%, and infection intensities were 24.9, 96.3, and 201.1 PFI, respectively. The SI of MsMc was 11.93, 64.42, and 135.14 in PFS from the upper, middle, and lower reaches of Seomjin-gang. The SI of MsMc were 17.08, 32.42, and 89.39 in
Zacco spp. from the upper, middle, and lower reaches of Seomjin-gang in this study.
These results collectively demonstrated that the endemicity of MsMc is relatively low in fish from the upper (Imsil-gun, Sunchang-gun, and Namwon-si, Jeollabuk-do) and middle reaches (Gokseong-gun and Gurye-gun in Jeollanam-do) of Seomjin-gang compared with that in fish from the lower reaches of Seomjin-gang (Hadong-gun, Gyeongsangnam-do). The individual endemicity with MsMc is different to some extent by the survey regions, but it evidenced a tendency to gradually increase along with the lower level of reaches in Seomjin-gang.
Nakdong-gang
The prevalence of MsMc was 49.2%, 41.9%, 36.6%, 39.9%, 22.9%, 23.8%, 37.9%, 87.5%, and 57.7% in PFS from ㉝ Banbyeon-cheon, ㉞ Yongjeon-cheon, ㉟ Wi-cheon (in 3 times of survey), ㊱
Yang-cheon (in the former and the latter survey), ㊲ Deokcheon-gang, and Jisu-cheon (+Haman-cheon) (
Table 2). The infection intensities were 3.4, 14.5, 3.8, 3.9, 28.4, 7.1, 5.0, 228.2, and 6.4 PFI, respectively. The infection status by the fish species and survey locality is shown in
Supplementary Tables S10–
S13.
It has been known that the riverside area of Nakdong-gang is an endemic area of clonorchiasis in Korea [
1–
7]. The high endemicity of
C. sinensis metecercariae was also reported in fish from the water systems of Nakdong-gang [
66]. However, to date, only a limited number of studies on the endemicity of MsMc have been conducted in this watershed, and no endemic areas of metagonimiasis have been reported in these regions. A previous study investigated the infections of DTM in freshwater fish from Geumho-gang in Gyeongsangbuk-do [
32]. MsMc were detected in 234 (47.9%) out of 489 fish in 10 PFS. As a result of investigating the status of MsMc infection in sweet smelt and sea rundace in 3 regions of Osip-cheon, Hyeongsan-gang, and Yu-cheon in Gyeongsangbuk-do, the infection intensity of MsMc was found to be 4,333 (328–12,767) and 1,541 (408–2,652) PFI in 10 each of sweet smelt and sea rundace, respectively [
34].
The endemicity of MsMc was relatively low in 8 survey regions of Nakdong-gang except for Deokcheon-gang in Sancheong-gun, Gyeongsangnam-do. The endemic status of MsMc in Deokcheon-gang was preiously reported [
62]. In this study, the prevalence of MsMc was 43.0%, 31.9%, and 46.8% in PFS from the upper (Banbyeon-cheon and Yongjeon-cheon), middle (Wi-cheon), and lower (Yang-cheon, Deokcheon-gang, and Jisu-cheon+Haman-cheon) reaches. Their infection intensities were 12.6, 11.3, and 123.0 PFI, repectively. The SI of MsMc were 5.40, 3.61, and 57.56 in PFS. The SI in
Zacco spp. was 13.22, 1.97, and 28.4 in the upper, middle, and lower reaches of Nakdong-gang.
Three streams Banbyeon-cheon, Yongjeon-cheon, and Wi-cheon that flow in Yeongyang-gun, Cheongsong-gun, and Gunwi-gun flow into the inland of Gyeongsangbuk-do, where the upper Nakdong-gang flows. The endemicity of MsMc were very low from these stream fish. Even for the index fish,
Zacco spp., SI was very low in these 3 streams. The survey region of Gyeongho-gang, which is composed of branch streams, Yang-cheon and Deokcheon-gang (in Sancheong-gun), and of Nam-gang (Jisu-cheon and Haman-cheon) are the lower reaches of Nakdong-gang in Gyeongsangnam-do. The endemicity of MsMc were very low in all survey regions except for Deokcheon-gang. The SI of index fish was high only in Deokcheon-gang and very low in the other 3 regions [
62]. Collectively, the endemicity of MsMc is relatively low in fish from the water systems of Nakdong-gang except for Deokcheon-gang, while these regions are known to be high endemic areas of clonorchiasis. Ecological environment is different between 2 fluke groups,
Metagonimus spp. and
C. sinensis. The first intermediate snail host of
Metagonimus spp. (
Semisulcospira spp.) mainly thrives clean, cold torrents and streams in the upper and middle reaches of rivers, whereas
Parafossarulus manchouricus, the snail host of
C. sinensis commonly inhabits stagnant water such as lake, pond, rice paddy, and irrigation ditchs.
The prevalence of MsMc was 78.1%, 56.8%, 85.4%, 59.3%, 48.5%, and 38.4% in PFS from ㊴ Namdae-cheon, ㊵ Osip-cheon (Samcheok), ㊶ Whangpi-cheon, ㊶ Osip-cheon (Yeongdeok), ㊸ Gigye-cheon, and ㊹ Taehwa-gang (
Table 2). The infection intensities were 263.0, 41.8, 307.0, 33.6, 3.4, and 110.2 PFI, respectively. The infection status by the fish species and survey locality is presented in
Supplementary Table S14.
Many Korean workers have surveyed the epidemiological situation of metagonimiasis and the infection status of MsMc in fish hosts including sweet smelt in the east coastal areas of Korea [
2,
17–
19]. Previous studies reported 13.3% prevalence among 1,172 inhabitants in Samcheok-si, Gangwon-do, and 6.6% prevalence from 2,357 inhabitants in the eastern coast of Gangwon-do [
17,
18]. Another study also found high endemicity of metagonimiasis among residents of Samcheok-si, Gangwon-do, in which 29.7% prevalence from 165 residents examined. Worm burden was over 22,000 in average from 11 candidate residents [
19]. Since Choi et al. [
30] first surveyed the infection with MsMc in sea rundace,
T. hakonensis, from the lower reaches of Hyeongsan-gang in Gyeongsangbuk-do, Ahn [
44] reported MsMc infections in
T. hakonensis from Osip-cheon in Samcheok-si. Joo and his colleagues investigated the infections of DTM including MsMc in fish from the streams of Hyeongsan-gang and Taehwa-gang in Gyeongsangbuk-do and Ulsan Metropolitan City [
67–
70]. The infection status of MsMc has been frequently examined in
P. altivelis, which is the most susceptible fish host in streams along the east coastal areas of Korea [
17,
18,
37,
38,
41,
43,
44,
48,
49]. Recently, Sohn et al. [
58] surveyed DTM in fish from 5 coastal lakes, i.e., Hwajinpo-ho, Songji-ho, Mae-ho, Hyang-ho, and Gyeongpo-ho in Gangwon-do. They detected MsMc in 52 (41.3%) of 126
T. hakonensis and their infection intensity was 14.6 PFI. It has been known that coastal lakes in Gangwon-do have an ecologically favorable environmental condition for brackish water fish. Therefore, this fish ecology is regarded as a good factor for studies on the heterophyid flukes including the species diversity of
Metagonimus in Korea.
In this review, MsMc prevalence was relatively high in fish from Whangpi-cheon (85.4%) and Namdae-cheon (78.1%). The infection intensities were higher in fish from Whangpi-cheon (307 PFI), Namdae-cheon (263 PFI), and Taehwa-gang (110 PFI). The endemicity was also high in fish from Whangpi-cheon (180.06) and Namdae-cheon (174.37). High endemicity of MsMc was probably caused by the number of highly susceptible fish species, P. altivelis, T. hakonensis, and Onchorhynchus masou in these 2 regions. Interstingly, however, the high infection intensity was found to be caused by 20 Chinese minnow, Rhynchocypris oxycephalus, as well as 16 sweet smelt in Taehwa-gang. In rivers along the east coast of Korea, the SI of MsMc in the index fish Zacco spp. was relatively low, but that in Namdae-cheon fish showed moderate level (42.58).
Endemicity of MsMc in fish by survey areas
The endemicities of MsMc in fish from water systems of ② Hantan-gang (Yeoncheon) (2.02) and ④ Imjin-gang (2.90), which were very low, while those from ① Hantan-gang (Cheorwon) (14.48) and ③ Togyo-jeosuji (36.25) were low and moderate. In the water systems of Han-gang, endemicities were low in fish from ⑤ Pyeongchang-gang (5.44), ⑧ Seom-gang (Wonju) (8.80), and ⑦ Seom-gang (Hoengseong) (9.56). However, endemicities in fish from ⑨ Dal-cheon (29.01) and ⑥ Joyang-gang (31.15) was moderate. The endemicities of MsMc were very low in fish from ⑫ Geum-gang (Muju) (0.17), ⑭ Yugu-cheon (0.41), ⑯ Nonsan-cheon (0.45), and ⑩ Juja-cheon (0.68). However, endemicities in fish from ⑪ Chogang-cheon (3.89), ⑮ Ji-cheon (13.99), and ⑬ Geum-gang (Geumsan) (17.78) were low and moderate. The endemicity of MsMc showed very low and low levels in fish from ⑰ Mangyeong-gang (0.89 and 4.17). In the water systems of Yeongsan-gang, endemicities were very low in fish from ⑱ Hwangryong-gang (0.94) and ⑲ Jiseok-cheon (1.48), while that of ⑳ Yeongam-cheon was high (91.22). The endemicities of MsMc in fish from Tamjin-gang were moderate and high (㉑-2 (Jangheung) 25.44 and ㉒ (Gangjin) 65.54, and ㉑-1 (Jangheung) 85.25, respectively).
In the water systems of Seomjin-gang, endemicities were very low and low in fish from ㉓ Osu-cheon (0.52), ㉔-2 Seomjin-gang (Sunchang) (2.65), ㉜ Jugyo-cheon (4.97), ㉕ Songdae-cheon (9.19), and ㉖-2 Seomjin-gang (Gokseong) (13.76). However, moderate and high endemicities were found in fish from ㉔-1 Seomjin-gang (Sunchang) (26.91), ㉚ Namsan-cheon (27.83), ㉖-1 Seomjin-gang (Gokseong) (28.86), ㉙ Akyang-cheon (66.56), ㉛ Hoeng-cheon (79.17), ㉗ Seomjin-gang (Gurye) (143.34), and ㉘ Hwagye-cheon (508.40), respectively. The endemicity of MsMc in fish from the water systems of Nakdong-gang was relatively low except for that in ㊲ Deokcheon-gang (190.78). On the other hand, it was very low in fish from ㉟-1 Wi-cheon (0.59), ㉟-2 Wi-cheon (0.86), ㉝ Banbyun-cheon (0.97), ㊱-1 Yang-cheon (1.11), ㊳ Jisu-cheon and Haman-cheon (1.41), and ㊱-2 Yang-cheon (1.65). The low endemicity was observed in fish from ㉟-3 Wi-cheon (3.59) and ㉞ Yongjeon-cheon (3.65). In streams of the east coastal areas, endemicities in fish from ㊴ Namdae-cheon (174.37) and ㊶ Wangpi-cheon (180.06) were high, while those from ㊹ Cheokgwa-cheon and Taehwa-gang (22.19), ㊵ Osip-cheon (Samcheok) (12.63), ㊵ Osip-cheon (Yeongdeok) (11.70), and ㊸ Gigye-cheon (0.97) were relatively low (
Table 3). Collectively, the endemicity of MsMc was high in fish from 8 (15.7%) regions, i.e., ⑳ Yeongam-cheon, ㉑-1 Tamjin-gang (Jangheung), ㉗ Seomjin-gang (Gurye), ㉘ Hwagye-cheon, ㉑ Hoeng-cheon, ㊲ Deokcheon-gang, ㊴ Namdae-cheon, and ㊶ Whangpi-cheon. The moderate levels were observed in fishes from 9 (17.6%) survey regions, i.e., ③ Togyo-jeosuji, ⑥ Joyang-gang, ⑨ Dal-cheon, ㉑-2 Tamjin-gang (Jangheung),㉒ Tamjin-gang (Gangjin), ㉔-1 Seomjin-gang (Sunchang), ㉖-1 Seomjin-gang (Gokseong),㉙ Akyang-cheon, and ㉚ Namsan-cheon. Fish from 16 (31.4%) locality showed low endemicity, which included ① Hantan-gang (Cheorwon), ⑤ Pyeongchang-gang, ⑦ Seom-gang (Hoengseong), ⑧ Seom-gang (Wonju), ⑪ Chogang-cheon, ⑬ Geum-gang (Geumsan), ⑮ Ji-cheon, ⑰-2 Soyang-cheon, ㉕ Songdae-cheon, ㉖-2 Seomjin-gang (Gokseong), ㉜ Jugyo-cheon, ㉞ Yongjeon-cheon, ㉟-3 Wi-cheon, ㊵ Osip-cheon (Samcheok), ㊷ Osip-cheon (Yeongdeok), and ㊹ Taehwa-gang and Cheokgwa-cheon, respectively. The endemicity of MsMc was very low in 18 (35.3%) region fish, i.e., ② Hantan-gang (Yeoncheon), ④ Imjin-gang, ⑩ Juja-cheon, ⑫ Geum-gang (Muju), ⑭ Yugu-cheon, ⑯ Nonsan-cheon, ⑰-1 Soyang-cheon, ⑱ Hwangryong-gang, ⑲ Jiseok-cheon, ㉓ Osu-cheon, ㉔-2 Seomjin-gang (Sunchang), ㉝ Banbyeon-cheon, ㉟-1 Wi-cheon, ㉟-2 Wi-cheon, ㊱-1 Yang-cheon, ㊱-2 Yang-cheon, ㊳ Jisu-cheon, and ㊸ Gigye-cheon (
Table 4).
Three chub species,
Z. platypus,
Z. temminckii, and
Z. koreanus (
Fig. 3), which are widely distributed in the water systems in Korea, are appropriate to be the index fish to determine the endemicity of MsMc.
The SI of MsMc in index fish, Zacco spp., from water systems of Hantan-gang and Imjin-gang was ① Hantan-gang (Cheorwon) (30.09), ② Hantan-gang (Yeoncheon) (3.87), ③ Togyo-jeosuji (131.24), and ④ Imjin-gang (13.19), respectively. In the water systems of Han-gang (⑤–⑨), SI was found to be 10.88, 106.33, 23.80, 45.98, and 80.67, respectively. In Geum-gang water system, the SI showed very low level at 0.27 (⑫ Geum-gang in Muju), 1.82 (⑭ Yugu-cheon), and 3.26 (⑩ Juja-cheon). It was low at 5.38 (⑮ Ji-cheon) and moderate at 43.70 (⑬ Geum-gang in Geumsan) and 74.0 (⑪ Chogang-cheon), respectively. No chub fish was examined in ⑯ Nonsan-cheon. The SI was very low and low in fish from ⑰ Mangyeong-gang (2.72 and 25.97). The SI of Yeongsan-gang tributaries was low at 8.77 and 5.24 in ⑱ (Hwangryong-gang) and ⑲ (Jiseok-cheon), while it was high at 283.77 in ⑳ (Yeongam-cheon). The SI of Tamjin-gang tributaries ㉑-1 (Jangheung: 30.02), ㉒ (Gangjin: 32.93), and ㉑-2 (Jangheung: 65.18) were at a moderate levels.
The SI in Seomjin-gang was very low in ㉓ Osu-cheon (2.22), low in ㉔-2 Seomjin-gang (Sunchang) (8.22), ㉜ Jugyo-cheon (9.97), ㉖-1 Seomjin-gang (Gokseong) (18.36), ㉔-1 Seomjin-gang (Sunchang) (18.90), and ㉖-2 Seomjin-gang (Gokseong) (26.98); moderate in ㉕ Songdae-cheon (39.37), ㉘ Hwagye-cheon (43.37), ㉗ Seomjin-gang (Gurye) (65.18), ㉚ Namsan-cheon (72.88), and ㉙ Akyang-cheon (76.55); and high in ㉛ Hoeng-cheon (192.27). The SI of MsMc in index fish from Nakdong-gang was relatively low except for in ㊲ Deokcheon-gang (119.07). It was very low in
Zacco spp. from ㉟-3 Wi-cheon (1.05), ㉟-1 Wi-cheon (2.37), ㉟-2 Wi-cheon (2.45), ㊱-2 Yang-cheon (2.47), ㉝ Banbyun-cheon (2.78), ㊱-1 Yang-cheon (2.95), and ㊳ Jisu-cheon and Haman-cheon (3.12), respectively. The low SI was shown in index fish from ㉞ Yongjeon-cheon (16.14). In streams of the east coastal areas, the SI in fish from ㊸ Gigye-cheon (1.40), ㊹ Cheokgwa-cheon and Taehwa-gang (1.76), and ㊶ Wangpi-cheon (3.88) was very low, while it was low and moderate in ㊷ Osip-cheon (Yeongdeok) (7.73), ㊵ Osip-cheon (Samcheok) (11.96), and ㊴ Namdae-cheon (42.58) (
Table 5).
Collectively, the SI in index fish,
Zacco spp., is fairly high in 5 (10.0%) survey regions, i.e., ③ Togyo-jeosuji, ⑥ Joyang-gang, ⑳ Yeongam-cheon, ㉛ Hoeng-cheon, and ㊲ Deokcheon-gang. The moderate SI levels were observed in fishes from 14 (28.0%) survey regions, i.e., ① Hantan-gang (Cheorwon), ⑧ Seom-gang (Wonju), ⑨ Dal-cheon, ⑪ Chogang-cheon, ⑬ Geum-gang (Geumsan), ㉑-1, and ㉑-2 Tamjin-gang (Jangheung), ㉒ Tamjin-gang (Gangjin), ㉕ Songdae-cheon, ㉗ Seomjin-gang (Gurye), ㉘ Hwagyae-cheon, ㉙ Akyang-cheon, ㉚ Namsan-cheon, and ㊴ Namdae-cheon. The low SI was shown in index fish from 15 (30.0%) regions, i.e., ④ Imjin-gang, ⑤ Pyeongchang-gang, ⑦ Seom-gang (Hoengseong), ⑮ Ji-cheon, ⑰-2 Soyang-cheon, ⑱ Hwangryong-gang, ⑲ Jiseok-cheon, ㉔-1 and -2 Seomjin-gang (Sunchang), ㉖-1 and ㉖-2 Seomjin-gang (Gokseong), ㉜ Jugyo-cheon, ㉞ Yongjeon-cheon, ㊵ Osip-cheon (Samcheok), and ㊷ Osip-cheon (Yeongdeok). The SI was very low in 16 (32.0%) regions, i.e., ② Hantan-gang (Yeoncheon), ⑩ Juja-cheon, ⑫ Geum-gang (Muju), ⑭ Yugu-cheon, ⑰-1 Soyang-cheon, ㉓ Osu-cheon, ㉝ Banbyeon-cheon, ㉟-1, ㉟-2 and ㉟-3 Wi-cheon, ㊱-1 and ㊱-2 Yang-cheon, ㊳ Jisu-cheon, ㊶ Whangpi-cheon, ㊸Gigye-cheon, and ㊹ Taehwa-gang (+Cheokgwa-cheon), respectively (
Table 6).
The sweet smelt,
P. altivelis (
Fig. 4), has been known as the main infection source of human metagonimiasis in the endemic areas of Korea. This fish species has been popularly eaten in raw by the residents in the eastern and southern endemic areas. The epidemiological studies on the infections of MsMc in sweet smelt have been conducted by many workers in east and south coastal areas of Korean peninsula [
14–
18,
27,
29,
33–
35,
37,
38,
41,
43,
48]. However, these studies have not been currently conducted in Korea. In this review, the recent infection status of MsMc was analyzed in 346 sweet smelt in 13 survey regions.
In Tamjin-gang, MsMc were detected 93 (98.9%) out of 94 sweet smelt in 2 survey regions, Jangheung-gun (53/54; 98.1%) and Gangjin-gun (40/40). Their infection intensity was 1,125 PFI (1,339 and 841 PFI, respectively). MsMc were found in 34 (75.6%) out of 45 sweet smelt from 5 survey regions (Sunchang, Gokseong, Gurye, Hwagye-cheon, and Akyang-cheon) in Seomjin-gang branches. Their overall infection intensity was 5,421 PFI (3,146, 826, 8,563, 6,830, and 4,215 PFI, respectively). In sweet smelt from Deokcheon-gang, an inland branch stream of Nakdong-gang, MsMc were detected in 40 (97.6%) out of 41 fish examined. The infection intensity averaged 3,570 PFI. MsMc were found 157 (94.6%) out of 166 sweet smelt from 5 survey regions, i.e., Namdae-cheon, Osip-cheon (Samcheok), Whangpi-cheon, Osip-cheon (Yeongdeok), and Taehwa-gang, in the east coastal areas. Their overall infection intensity was 558 MsMc PFI (1,522, 18, 939, 63, and 331 PFI, respectively). The infection status of MsMc in the most susceptible fish host,
P. altivelis, by the survey localities was presented in
Table 7.
Except for the highly susceptible fish species,
P. altivelis, MsMc were detected in 9 fish species, i.e., 3
Zacco spp.,
O. uncirostris,
Rhynchocypris oxycephalus,
C. auratus,
Acheilognathus rhombeus,
O. masou, and
T. hakonensis. Infection status of MsMc by the fish species and by survey localities are summarized in
Table 8.
Metacercaria of
Metagonimus spp. was firstly described from crusian carp,
C. auratus in 1917 [
25]. Thereafter, many workers reported the fish intermediate hosts of
Metagonimus spp. in Korea [
22,
23,
26–
28,
30,
32,
39,
40,
47,
65,
71–
74]. Sohn [
26] collectively arranged the fish intermediate hosts of
Metagonimus spp. and nominated 44 fish species in 6 families (Cyprinidae: 32 spp., Amblycipitidae: 1 sp., Bagridae: 2 spp., Centrarchidae: 1 sp., Cobitidae: 2 spp., Gobiidae: 1 sp., Lateolabracidae: 1 sp., Osphronemidae: 1 sp., Plecoglossidae: 1 sp., and Sinipercidae: 2 spp.). Recently, we added 30 fish species, which included
Acanthorhodeus macropterus,
Acheilognathus koreensis,
A. majusculus,
A. signifer,
A. somjinensis,
Hemibarbus mylodon,
Hemiculter leucisculus,
Ladislabia taczanowskii,
Microphysogobio koreensis,
M. longidorsalis,
Pseudopuntungia tenuicorpa,
Rhodeus pseudosericeus,
Squalidus japonicus coreanus,
S. multimaculatus, Zacco koreanus, Liobagrus andersoni, L. somjinensis, Pseudobagrus koreanus, Channa argus, Micropterus salmoides, Cobitis sinensis, Koreocobitis naktongensis, Cottus hangiogensis, Acanthogobius lactipes, Gymnogobius urotaenia, Tridentiger brevispinis, Mugil cephalus, Odontobutis interrupta, O. platycephala, and
Oncorhynchus masou masou, in the list of the second intermediate hosts [
49–
51,
57–
62]. Among them, 3 gobiid fish species, i.e.,
A. lactipes, G. urotaenia, and
T. brevispinis, are mainly inhabit in brackish water. These fish species were reported as the second intermediate hosts of
M. otsurui in Japan [
75–
77]. However, no MsMc were found in a total of 239 fish in 7 gobiid spp., i.e.,
Acanthogobius flavimanus (
n=76),
A. lactipes (42),
Tridentiger brevispinis (44),
T. trigonocephalus (11),
T. obscurus (10),
Gymnogobius castaneus (41), and
Favonigobius gymnauchen (15), from 5 coastal lakes in Gangwon-do. MsMc were mainly detected in sea rundace,
T. hakonensis [
58]. As summarized in
Table 9, 74 fish species in 15 families are currently designated as the second intermediate hosts of
Metagonimus spp. in Korea.
Metagonimiasis is one of the fish-borne trematodiasis, which is still endemic in Korea. This endemic disease causes a significant public health concern among residents in some major river basins. The infection status of MsMc in fish hosts is intimately associated with transmission of human disease. This article reviewed data from 10 years (2011–2020) of our research. The infection status of MsMc was analyzed for 19,568 fish of 87 species collected from 9 main water systems in Korea, such as Hantan-gang and Imjin-gang, Han-gang, Geum-gang, Mangyeong-gang, Yeongsan-gang, Tamjin-gang, Seomjin-gang, Nakdong-gang, and streams in the east coastal areas. This study clarifies some of the characteristics of MsMc infection in fish: The high endemicity was observed in fish from middle and low reaches of Seomjin-gang, Tamjin-gang (Jangheung-gun in Jeollanam-do), Deokcheon-gang (Sancheong-gun in Gyeongsangnam-do), Namdae-cheon (Yangyang-si in Gangwon-do), Whangpi-cheon (Uljin-gun in Gyeongsangbuk-do), and Yeongam-cheon (Yeongam-gun in Jeollanam-do). The high endemicity of MsMc depends on the number of susceptible fish species, especially sweet smelt, P. altivelis, and some other species. The infection status of MsMc in index fish, Zacco spp., might represent the overall infection patterns of the fish hosts. The susceptibility of Zacco spp. to MsMc was found to be very low and low in 62.0% of the areas, and moderate and high in 38.0% of the regions. This study also analyzed recent infection status of MsMc, which is the most susceptible fish host, P. altivelis. Except for the sweet smelt, 9 fish species, i.e., Z. platypus, Z. koreanus, Z. temminckii, O. uncirostris, R. oxycephalus, C. auratus, A. rhombeus, O. masou, and T. hakonensis showed the higher infections with MsMc in some survey regions. In Korea, 74 species in 15 families of fish have been collectively registered as second intermediate hosts of Metagonimus spp.
Nowadays, the endemicity of MsMc infections in fish hosts and the incidence of human metagonimiasis are gradually decreasing in this country. However, continuous monitoring of infection status of fish hosts is required to control and manage metagonimiasis affecting humans and reservoir hosts. In faunistic view point, studies on the species diversity of Metagonimus fluke including previously reported 3 spp. should be performed with ecologically different worm samples by the biological, morphological, and molecular approaches in Korea.
Notes
-
Conceptualization: Sohn WM
Data curation: Sohn WM
Formal analysis: Sohn WM
Investigation: Sohn WM
Methodology: Sohn WM
Project administration: Sohn WM
Resources: Sohn WM
Software: Sohn WM
Supervision: Sohn WM
Validation: Sohn WM
Visualization: Sohn WM
Writing – original draft: Sohn WM
Writing – review & editing: Sohn WM
-
The author has no conflicts of interest concerning the work reported in this paper.
Supplementary Information
Supplementary Table S1.
Infection status of Metagonimus spp. metacercariae (MsMc) in fish from the water systems of Hantan-gang and Imjin-gang in Korea
Supplementary Table S4.
Infection status of Metagonimus spp. metacercariae (MsMc) in fish from Soyang-cheon (stream) in Wanju-gun, Jeollabuk-do, Korea
Supplementary Table S5.
Infection status of Metagonimus spp. metacercariae (MsMc) in fish from the water systems of Yeongsan-gang in Jeollanam-do, Korea
Supplementary Table S8.
Infection status of Metagonimus spp. metacercariae (MsMc) in fish from Seomjin-gang (middle reaches) in Jeollanam-do, Korea
Supplementary Table S9.
Infection status of Metagonimus spp. metacercariae (MsMc) in fish from Seomjin-gang (lower reaches) in Hadong-gun, Gyeongsangnam-do, Korea
Supplementary Table S10.
Infection status of Metagonimus spp. metacercariae (MsMc) in fish from the water systems of Nakdong-gang in Gyeongsangbuk-do, Korea
Supplementary Table S11.
Infection status of Metagonimus spp. metacercariae (MsMc) in fish from the water systems of Nakdong-gang in Gyeongsangbuk-do, Korea
Supplementary Table S12.
Infection status of Metagonimus spp. metacercariae (MsMc) in fish from the water systems of Nakdong-gang in Gyeongsangnam-do, Korea
Supplementary Table S13.
Infection status of Metagonimus spp. metacercariae (MsMc) in fish from the water systems of Nakdong-gang in Gyeongsangnam-do, Korea
Supplementary Table S14.
Infection status of Metagonimus spp. metacercariae (MsMc) in fish from the water systems in the eastern coastal regions of Korea
Acknowledgment
I thank Jung-A Kim and Hee-Joo Kim of the Department of Parasitology and Tropical Medicine, Gyeongsang National University College of Medicine, for their sincere help in the examination of fish.
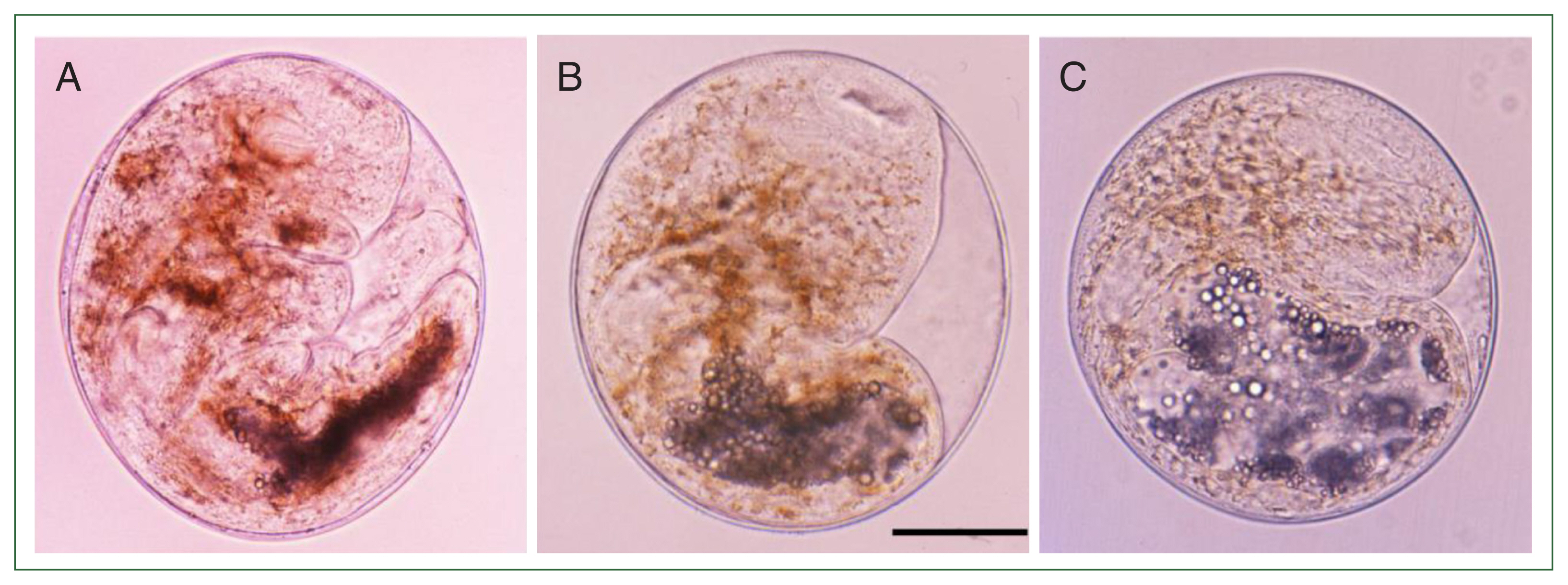
Fig. 1Metacercariae of Metagonimus spp. in different fish hosts, Plecoglossus altivelis (A), Carassius auratus (B), and Zacco platypus (C) in Korea. The metacercariae are spherical or disc-shape and meaure 145–172 (160)×125–158 (140) μm. They had yellow brownish pigment granules, a ventral sucker deflectively located from median and a V-shaped excretory bladder. Scale bar=50 μm.
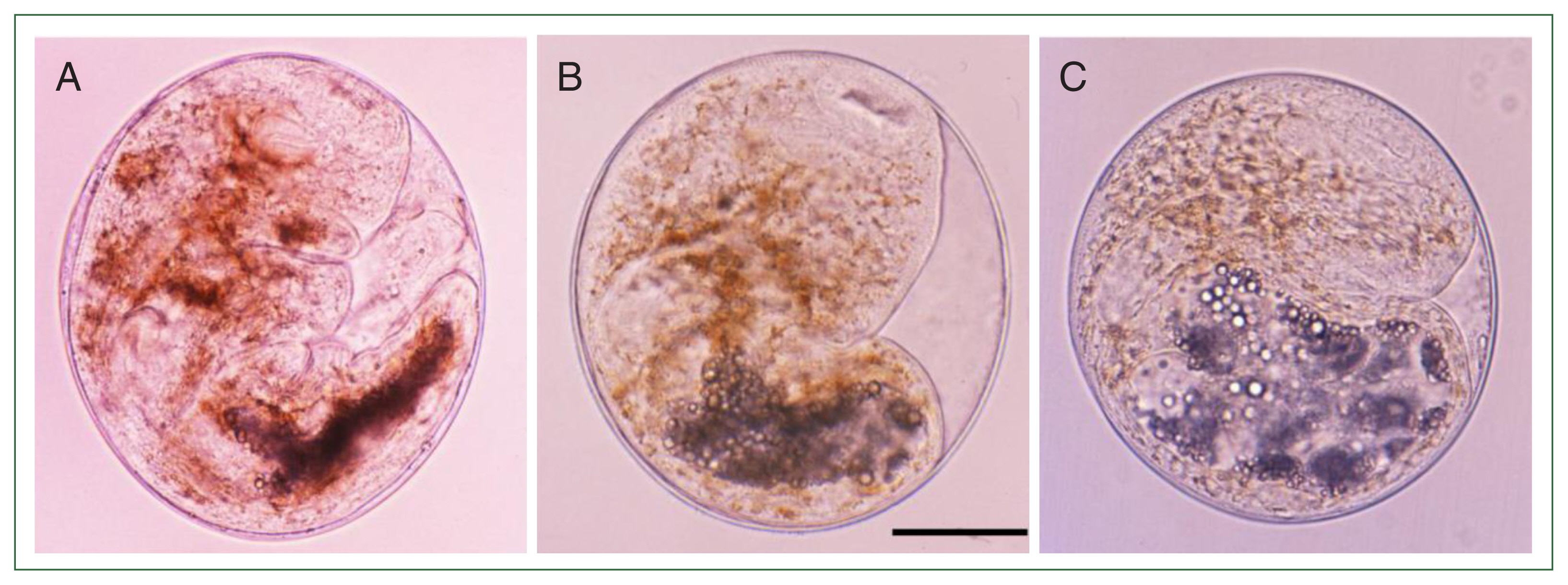
Fig. 2A map showing 44 surveyed localities. Detailed information of each site (No., water system and administrative region) is shown in
Table 1.
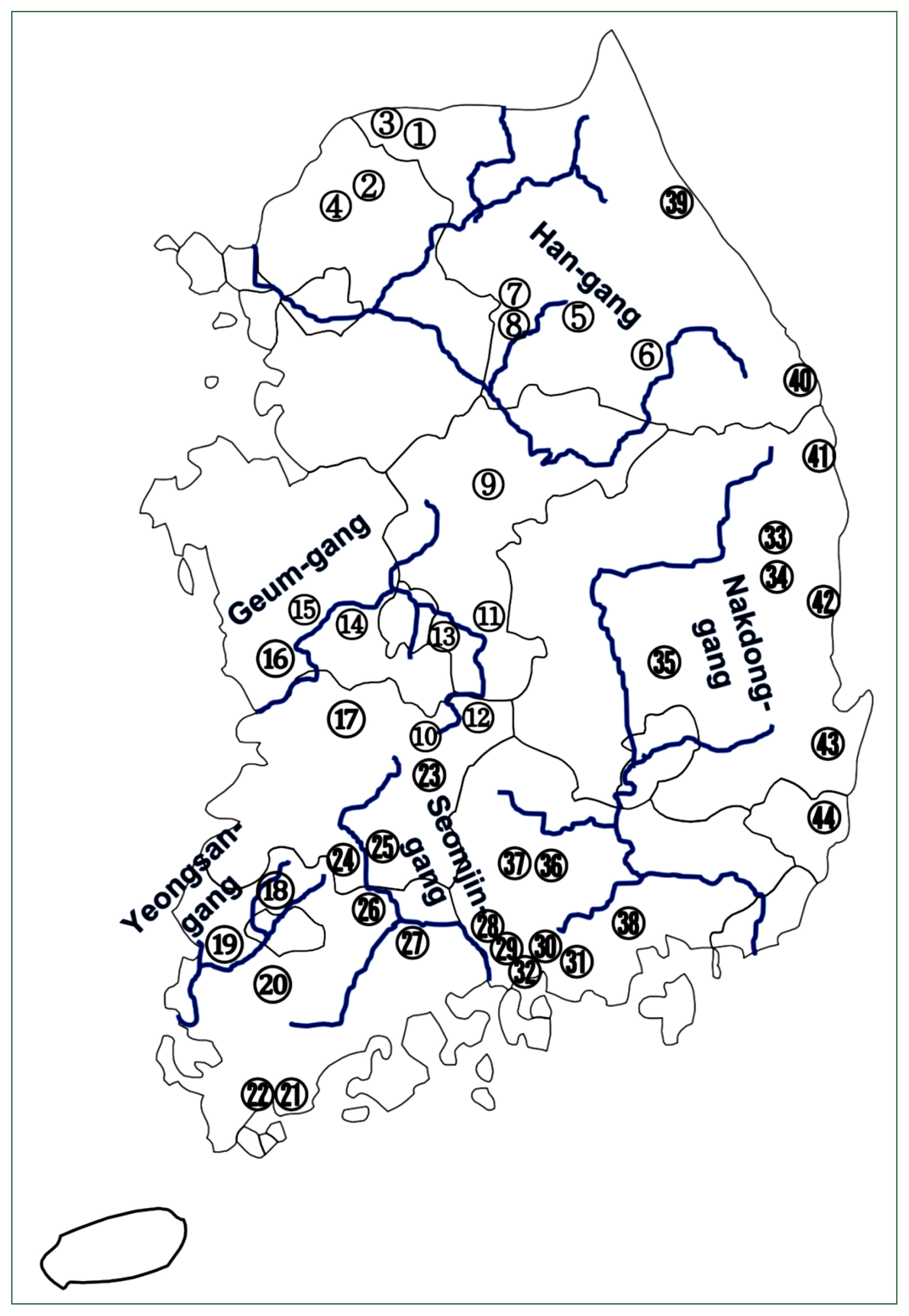
Fig. 3The index fish, Zacco platypus (A), Z. temminckii (B), and Z. koreanus (C). Male (top) and female (bottom), respectively. They are relatively susceptible to Metagonimus spp. metacercaria and widely distributed in Korean waters. Bar=5 cm.

Fig. 4Sweet smelt, Plecoglossus altivelis, the most susceptible and heavily infected fish with Metagonimus spp. metacercariae. The fish is common in rivers and streams on the east and south coasts of Korea. Bar=5 cm.

Table 1Summary on the fishes
a examined for
Metagonimus spp. metacercariae by the survey localities
Table 1
|
Locality |
No. fish spp. examineda
|
No. fish examined (No. infected fish (NIF))b
|
|
No. of water system |
Administrative region |
|
① Hantan-gang |
Cheorwon-gun, Gangwon |
32 |
540 (130) |
|
② Hantan-gang |
Yeoncheon-gun, Gyeonggi |
13 |
195 (63) |
|
③ Togyo-jeosuji |
Cheorwon-gun |
19 |
568 (158) |
|
④ Imjin-gang |
Gyeonggi-do |
15 |
283 (68) |
|
⑤ Pyeongchang-gang |
Pyeongchang-gun, Gangwon |
15 |
228 (54) |
|
⑥ Joyang-gang |
Jeongseon-gun, Gangwon |
15 |
196 (59) |
|
⑦ Seom-gang |
Hoengseong-gun, Gangwon |
20 |
184 (28) |
|
⑧ Seom-gang |
Wonju-si, Gangwon |
30 |
707 (148) |
|
⑨ Dal-cheon |
Goesan-gun, Chungbuk |
12 |
99 (30) |
|
⑩ Juja-cheon |
Jinahn-gun, Jeonbuk |
19 |
208 (80) |
|
⑪ Chogang-cheon |
Yeongdong-gun, Chungbuk |
15 |
132 (6) |
|
⑫ Geum-gang |
Muju-gun, Jeonbuk |
14 |
123 (11) |
|
⑬ Geum-gang |
Geumsan-gun, Chungnam |
25 |
365 (60) |
|
⑭ Yugu-cheon |
Gongju-si, Chungnam |
14 |
311 (59) |
|
⑮ Ji-cheon |
Cheongyang-gun, Chungnam |
89 |
89 (33) |
|
⑯ Nonsan-cheon |
Nonsan-si, Chungnam |
11 |
44 (0) |
|
⑰ -1 Soyang-cheon |
Wanju-gun, Jeonbuk |
18 |
607 (128) |
|
⑰ -2 Soyang-cheon |
Wanju-gun |
25 |
465 (109) |
|
⑱ Hwangryong-gang |
Jangseong-gun, Jeonnam |
14 |
107 (15) |
|
⑲ Jiseok-cheon |
Naju-si, Jeonnam |
14 |
140 (50) |
|
⑳ Yeongam-cheon |
Yeongam-gun, Jeonnam |
8 |
45 (19) |
|
㉑-1 Tamjin-gang |
Jangheung-gun, Jeonnam |
21 |
712 (167) |
|
㉑-2 Tamjin-gang |
Jangheung-gun |
25 |
972 (233) |
|
㉒ Tamjin-gang |
Gangjin-gun, Jeonnam |
17 |
517 (101) |
|
㉓ Osu-cheon |
Imsil-gun, Jeonbuk |
15 |
341 (100) |
|
㉔-1 Seomjin-gang |
Sunchang-gun, Jeonbuk |
29 |
310 (66) |
|
㉔-2 Seomjin-gang |
Sunchang-gun, Jeonbuk |
32 |
676 (147) |
|
㉕ Songdae-cheon |
Namwon-si, Jeonbuk |
25 |
420 (119) |
|
㉖-1 Seomjin-gang |
Gokseong-gun, Jeonnam |
15 |
289 (95) |
|
㉖-2 Seomjin-gang |
Gokseong-gun, Jeonnam |
22 |
631 (218) |
|
㉗ Seomjin-gang |
Gurye-gun, Jeonnam |
28 |
356 (77) |
|
㉘ Hwagye-cheon |
Hadong-gun, Gyeongnam |
15 |
202 (70) |
|
㉙ Akyang-cheon |
Hadong-gun |
12 |
179 (65) |
|
㉚ Namsan-cheon |
Hadong-gun |
14 |
328 (156) |
|
㉛ Hoeng-cheon |
Hadong-gun |
13 |
318 (125) |
|
㉜ Jugyo-cheon |
Hadong-gun |
23 |
196 (78) |
|
㉝ Banbyeon-cheon |
Yeongyang-gun, Gyeongbuk |
12 |
161 (64) |
|
㉞ Yongjeon-cheon |
Cheongsong-gun, Gyeongbuk |
20 |
804 (228) |
|
㉟-1 Wi-cheon |
Gunwi-gun, Gyeongbuk |
26 |
550 (204) |
|
㉟-2 Wi-cheon |
Gunwi-gun, Gyeongbuk |
33 |
723 (243) |
|
㉟-3 Wi-cheon |
Gunwi-gun, Gyeongbuk |
29 |
782 (227) |
|
㊱-1 Yang-cheon |
Sancheong-gun, Gyeongnam |
23 |
1,357 (322) |
|
㊱-2 Yang-cheon |
Sancheong-gun, Gyeongnam |
23 |
844 (241) |
|
㊲ Deokcheon-gang |
Sancheong-gun |
19 |
871 (180) |
|
㊳ Jisu-cheonc
|
Jinju-si, Gyeongnam |
13 |
167 (74) |
|
㊴ Namdae-cheon |
Yangyang-gun, Gangwon |
13 |
140 (38) |
|
㊵ Osip-cheon |
Samcheok-si, Gangwon |
15 |
143 (15) |
|
㊶ Whangpi-cheon |
Uljin-gun, Gyeongbuk |
13 |
239 (45) |
|
㊷ Osip-cheon |
Yeongdeok-gun, Gyeongbuk |
17 |
283 (67) |
|
㊸ Gigye-cheon |
Gyeongju-si, Gyeongbuk |
12 |
111 (41) |
|
㊹ Taehwa-gangd
|
Ulsan Metropilitan City |
17 |
310 (80) |
Table 2Overall infection status of Metagonimus spp. metacercariae (MsMc) in fish by water systems in Korea
Table 2
|
Locality surveyed |
No. fish examineda
|
No. (%) fish infected |
No. MsMc detected |
|
Range |
Average |
|
Hantan-gang and Imjin-gang |
|
① Hantan-gang (Cheorwon) |
512 |
283 (55.3) |
1–486 |
35.1 |
|
② Hantan-gang (Yeoncheon) |
177 |
60 (33.9) |
1–85 |
8.6 |
|
③ Togyo-jeosuji |
544 |
403 (74.1) |
1–1,020 |
62.0 |
|
④ Imjin-gang |
197 |
113 (57.4) |
1–250 |
9.6 |
|
|
Han-gang |
|
⑤ Pyeongchang-gang |
198 |
132 (66.7) |
1–145 |
10.0 |
|
⑥ Joyang-gang |
172 |
122 (70.9) |
1–403 |
60.1 |
|
⑦ Seom-gang (Hoengseong) |
93 |
69 (74.2) |
1–214 |
32.3 |
|
⑧ Seom-gang (Wonju) |
673 |
298 (44.3) |
1–374 |
27.4 |
|
⑨ Dal-cheon |
62 |
39 (62.9) |
1–329 |
79.4 |
|
|
Geum-gang |
|
⑩ Juja-cheon |
156 |
68 (43.6) |
1–25 |
4.2 |
|
⑪ Chogang-cheon |
89 |
41 (46.1) |
1–292 |
14.1 |
|
⑫ Geum-gang (Muju) |
60 |
15 (25.0) |
1–10 |
2.4 |
|
⑬ Geum-gang (Geumsan) |
261 |
205 (78.5) |
1–360 |
37.5 |
|
⑭ Yugu-cheon |
260 |
56 (21.5) |
1–14 |
2.9 |
|
⑮ Ji-cheon |
65 |
46 (69.2) |
1–332 |
32.7 |
|
⑯ Nonsan-cheon |
15 |
6 (40.0) |
1–13 |
4.2 |
|
|
Mangyeong-gang |
|
⑰ -1. Soyang-cheon |
490 |
174 (35.5) |
1–27 |
3.7 |
|
⑰ -2. Soyang-cheon |
424 |
152 (35.9) |
1–890 |
19.3 |
|
Yeongsan-gang |
|
⑱ Hwangryong-gang |
49 |
27 (55.1) |
1–26 |
5.9 |
|
⑲ Jiseok-cheon |
127 |
71 (55.9) |
1–25 |
5.3 |
|
⑳ Yeongam-cheon |
30 |
27 (90.0) |
1–1,520 |
202.7 |
|
|
Tamjin-gang |
|
㉑-1. Tamjin-gang (Jangheung) |
680 |
445 (65.4) |
1–5,320 |
152.5 |
|
㉑-2. Tamjin-gang (Jangheung) |
940 |
422 (48.1) |
1–5,280 |
63.1 |
|
㉒Tamjin-gang (Gangjin) |
491 |
326 (66.4) |
1–4,280 |
121.1 |
|
|
Seomjin-gang |
|
㉓Osu-cheon |
299 |
90 (30.1) |
1–32 |
3.7 |
|
㉔-1. Seomjin-gang (Sunchang) |
257 |
128 (49.8) |
1–6,750 |
86.8 |
|
㉔-2. Seomjin-gang (Sunchang) |
635 |
294 (46.3) |
1–130 |
7.1 |
|
㉕Songdae-cheon |
406 |
253 (62.3) |
1–334 |
21.8 |
|
㉖-1. Seomjin-gang (Gokseong) |
286 |
207 (72.4) |
1–4,380 |
43.1 |
|
㉖-2. Seomjin-gang (Gokseong) |
619 |
399 (64.5) |
1–833 |
25.0 |
|
㉗Seomjin-gang (Gurye) |
320 |
213 (66.6) |
1–16,830 |
281.5 |
|
㉘Hwagye-cheon |
194 |
141 (72.7) |
1–17,750 |
800.5 |
|
㉙Akyang-cheon |
156 |
132 (84.6) |
1–4,865 |
104.4 |
|
㉚Namsan-cheon |
323 |
185 (57.3) |
1–2,860 |
61.8 |
|
㉛Hoeng-cheon |
318 |
197 (61.9) |
1–5,860 |
127.7 |
|
㉜Jugyo-cheon |
174 |
128 (73.6) |
1–85 |
9.6 |
|
|
Nakdong-gang |
|
㉝Banbyeon-cheon |
132 |
65 (49.2) |
1–11 |
3.4 |
|
㉔Yongjeon-cheon |
730 |
306 (41.9) |
1–200 |
14.5 |
|
㉟-1. Wi-cheon |
399 |
146 (36.6) |
1–20 |
3.8 |
|
㉟-2. Wi-cheon |
566 |
226 (39.9) |
1–50 |
3.9 |
|
㉟-3. Wi-cheon |
708 |
162 (22.9) |
1–2,370 |
28.4 |
|
㊱-1. Yang-cheon |
1,282 |
305 (23.8) |
1–197 |
7.1 |
|
㊱-2. Yang-cheon |
813 |
308 (37.9) |
1–88 |
5.0 |
|
㊲Deokcheon-gang |
869 |
760 (87.5) |
1–20,650 |
228.2 |
|
㊳Jisu-cheon |
123 |
71 (57.7) |
1–49 |
6.4 |
|
|
Streams in east coastal areas |
|
㊴Namdae-cheon |
128 |
100 (78.1) |
1–6,280 |
263.0 |
|
㊵Osip-cheon (Samcheok) |
111 |
63 (56.8) |
1–638 |
41.8 |
|
㊶Whangpi-cheon |
226 |
193 (85.4) |
1–6,199 |
307.0 |
|
㊷Osip-cheon (Yeongdeok) |
246 |
146 (59.3) |
1–597 |
33.6 |
|
㊸Gigye-cheon |
103 |
50 (48.5) |
1–34 |
3.4 |
|
㊹Taehwa-gang |
255 |
98 (38.4) |
1–1,920 |
110.2 |
Table 3Endemicity of Metagonimus species metacercariae (MsMc) in fish by survey area in Korea
Table 3
|
Locality surveyed |
Positive ratea of fish species |
Positive rateb with MsMc in (+)ve fish sp. |
Mean no.c of MsMc detected |
Endemicityd
|
|
Hantan-gang and Imjin-gang |
|
① |
24/32 (0.75) |
283/512 (0.55) |
35.1 |
14.48 |
|
② |
9/13 (0.69) |
60/177 (0.34) |
8.6 |
2.02 |
|
③ |
15/19 (0.79) |
403/544 (0.74) |
62.0 |
36.25 |
|
④ |
8/15 (0.53) |
113/197 (0.57) |
9.6 |
2.90 |
|
|
Han-gang |
|
⑤ |
12/15 (0.80) |
132/198 (0.68) |
10.0 |
5.44 |
|
⑥ |
11/15 (0.73) |
122/172 (0.71) |
60.1 |
31.15 |
|
⑦ |
8/20 (0.40) |
69/93 (0.74) |
32.3 |
9.56 |
|
⑧ |
22/30 (0.73) |
298/673 (0.44) |
27.4 |
8.80 |
|
⑨ |
7/12 (0.58) |
39/62 (0.63) |
79.4 |
29.01 |
|
|
Geum-gang |
|
⑩ |
7/19 (0.37) |
68/156 (0.44) |
4.2 |
0.68 |
|
⑪ |
9/15 (0.60) |
41/89 (0.46) |
14.1 |
3.89 |
|
⑫ |
4/14 (0.29) |
15/60 (0.25) |
2.4 |
0.17 |
|
⑬ |
15/25 (0.60) |
205/261 (0.79) |
37.5 |
17.78 |
|
⑭ |
9/14 (0.64) |
56/260 (0.22) |
2.9 |
0.41 |
|
⑮ |
8/13 (0.62) |
46/65 (0.69) |
32.7 |
13.99 |
|
⑯ |
3/11 (0.27) |
6/15 (0.40) |
4.2 |
0.45 |
|
|
Mangyeong-gang |
|
⑰-1. |
12/18 (0.67) |
174/490 (0.36) |
3.7 |
0.89 |
|
⑰-2. |
15/25 (0.60) |
152/424 (0.36) |
19.3 |
4.17 |
|
|
Yeongsan-gang |
|
⑱ |
4/14 (0.29) |
27/49 (0.55) |
5.9 |
0.94 |
|
⑲ |
7/14 (0.50) |
71/127 (0.56) |
5.3 |
1.48 |
|
⑳ |
4/8 (0.50) |
27/30 (0.90) |
202.7 |
91.22 |
|
|
Tamjin-gang |
|
㉑-1. |
18/21 (0.86) |
445/680 (0.65) |
152.5 |
85.25 |
|
㉑-2. |
21/25 (0.84) |
422/940 (0.48) |
63.1 |
25.44 |
|
㉒ |
14/17 (0.82) |
326/491 (0.66) |
121.1 |
65.54 |
|
|
Seomjin-gang |
|
㉓ |
7/15 (0.47) |
90/299 (0.30) |
3.7 |
0.52 |
|
㉔-1. |
18/29 (0.62) |
128/257 (0.50) |
86.8 |
26.91 |
|
㉔-2. |
26/32 (0.81) |
294/636 (0.46) |
7.1 |
2.65 |
|
㉕ |
17/25 (0.68) |
253/406 (0.62) |
21.8 |
9.19 |
|
㉖-1. |
14/15 (0.93) |
207/288 (0.72) |
43.1 |
28.86 |
|
㉖-2. |
19/22 (0.86) |
399/624 (0.64) |
25.0 |
13.76 |
|
㉗ |
22/29 (0.76) |
213/320 (0.67) |
281.5 |
143.34 |
|
㉘ |
13/15 (0.87) |
141/194 (0.73) |
800.5 |
508.40 |
|
㉙ |
9/12 (0.75) |
132/156 (0.85) |
104.4 |
66.56 |
|
㉚ |
11/14 (0.79) |
185/323 (0.57) |
61.8 |
27.83 |
|
㉛ |
13/13 (1.0) |
197/318 (0.62) |
127.7 |
79.17 |
|
㉜ |
16/23 (0.70) |
128/174 (0.74) |
9.6 |
4.97 |
|
|
Nakdong-gang |
|
㉝ |
7/12 (0.58) |
65/132 (0.49) |
3.4 |
0.97 |
|
㉞ |
12/20 (0.60) |
306/730 (0.42) |
14.5 |
3.65 |
|
㉟-1. |
11/26 (0.42) |
146/399 (0.37) |
3.8 |
0.59 |
|
㉟-2. |
18/33 (0.55) |
226/566 (0.40) |
3.9 |
0.86 |
|
㉟-3. |
16/29 (0.55) |
162/708 (0.23) |
28.4 |
3.59 |
|
㊱-1. |
15/23 (0.65) |
305/1,280 (0.24) |
7.1 |
1.11 |
|
㊱-2. |
20/23 (0.87) |
308/813 (0.38) |
5.0 |
1.65 |
|
㊲ |
18/19 (0.95) |
760/869 (0.88) |
228.2 |
190.78 |
|
㊳ |
5/13 (0.38) |
71/123 (0.58) |
6.4 |
1.41 |
|
|
Streams in east coastal areas |
|
㊴ |
11/13 (0.85) |
100/128 (0.78) |
263.0 |
174.37 |
|
㊵ |
8/15 (0.53) |
63/111 (0.57) |
41.8 |
12.63 |
|
㊶ |
9/13 (0.69) |
193/226 (0.85) |
307.0 |
180.06 |
|
㊷ |
10/17 (0.59) |
146/246 (0.59) |
33.6 |
11.70 |
|
㊸ |
7/12 (0.58) |
50/103 (0.49) |
3.4 |
0.97 |
|
㊹ |
9/17 (0.53) |
98/255 (0.38) |
110.2 |
22.19 |
Table 4Distributions of endemicity with Metagonimus spp. metacercariae (MsMcs) in fish by the water systems in Korea
Table 4
|
Locality (River) surveyed |
No. of localities by the endemicitya
|
|
Very low |
Low |
Moderate |
High |
Total (%) |
|
Hantan-gang and Imjin-gang |
2 |
1 |
1 |
0 |
4 (7.8) |
|
Han-gang |
0 |
3 |
2 |
0 |
5 (9.8) |
|
Geum-gang |
4 |
3 |
0 |
0 |
7 (13.7) |
|
Mangyeong-gang |
1 |
1 |
0 |
0 |
2 (3.9) |
|
Yeongsan-gang |
2 |
0 |
0 |
1 |
3 (5.9) |
|
Tamjin-gang |
0 |
0 |
2 |
1 |
3 (5.9) |
|
Seomjin-gang |
2 |
3 |
4 |
3 |
12 (23.5) |
|
Nakdong-gang |
6 |
2 |
0 |
1 |
9 (17.6) |
|
Streams in east coast |
1 |
3 |
0 |
2 |
6 (11.8) |
|
Total (%) |
18 (35.3) |
16 (31.4) |
9 (17.6) |
8 (15.7) |
51 (100) |
Table 5Susceptibility index of the index fish Zacco spp. to Metagonimus spp. metacercariae (MsMc) according to the surveyed areas
Table 5
|
Surveyed areas |
No. fish examined |
No. (%) fish infected |
Mean no. MsMc detected |
Susceptibility indexa
|
|
Hantan-gang and Imjin-gang |
|
① |
130 |
123 (94.6) |
31.8 |
30.09 |
|
② |
63 |
28 (44.4) |
8.7 |
3.87 |
|
③ |
158 |
155 (98.1) |
133.8 |
131.24 |
|
④ |
68 |
49 (72.1) |
18.3 |
13.19 |
|
|
Han-gang |
|
⑤ |
54 |
52 (96.3) |
11.3 |
10.88 |
|
⑥ |
59 |
58 (98.3) |
108.2 |
106.33 |
|
⑦ |
28 |
24 (85.7) |
27.8 |
23.80 |
|
⑧ |
148 |
143 (96.6) |
47.6 |
45.98 |
|
⑨ |
30 |
27 (90.0) |
89.6 |
80.67 |
|
|
Geum-gang |
|
⑩ |
80 |
55 (68.8) |
4.7 |
3.26 |
|
⑪ |
6 |
6 (100) |
74.0 |
74.0 |
|
⑫ |
11 |
3 (27.3) |
1.0 |
0.27 |
|
⑬ |
60 |
55 (91.7) |
47.7 |
43.70 |
|
⑭ |
59 |
37 (62.7) |
2.9 |
1.82 |
|
⑮ |
33 |
20 (60.6) |
8.9 |
5.38 |
|
⑯ |
0 |
- |
- |
- |
|
|
Mangyeong-gang |
|
⑰-1. |
128 |
73 (57.0) |
4.8 |
2.72 |
|
⑰-2. |
109 |
91 (83.5) |
31.1 |
25.97 |
|
|
Yeongsan-gang |
|
⑱ |
15 |
14 (93.3) |
9.4 |
8.77 |
|
⑲ |
50 |
46 (92.0) |
5.7 |
5.24 |
|
⑳ |
19 |
19 (100) |
283.8 |
283.77 |
|
|
Tamjin-gang |
|
㉑-1. |
167 |
147 (88.0) |
34.1 |
30.02 |
|
㉑-2. |
233 |
210 (90.1) |
72.3 |
65.18 |
|
㉒ |
101 |
95 (94.1) |
35.0 |
32.93 |
|
|
Seomjin-gang |
|
㉓ |
100 |
57 (57.0) |
3.9 |
2.22 |
|
㉔-1. |
66 |
56 (84.8) |
22.3 |
18.90 |
|
㉔-2. |
147 |
108 (73.5) |
11.2 |
8.22 |
|
㉕ |
119 |
110 (92.4) |
42.6 |
39.37 |
|
㉖-1. |
95 |
84 (88.4) |
20.8 |
18.36 |
|
㉖-2. |
218 |
183 (83.9) |
32.2 |
26.98 |
|
㉗ |
77 |
65 (84.4) |
77.2 |
65.18 |
|
㉘ |
70 |
68 (97.1) |
44.7 |
43.37 |
|
㉙ |
65 |
65 (100) |
76.5 |
76.55 |
|
㉚ |
156 |
156 (100) |
72.9 |
72.88 |
|
㉛ |
125 |
117 (93.6) |
205.4 |
192.27 |
|
㉜ |
78 |
65 (83.3) |
12.0 |
9.97 |
|
|
Nakdong-gang |
|
㉝ |
64 |
46 (71.9) |
3.9 |
2.78 |
|
㉞ |
228 |
184 (80.7) |
20.0 |
16.14 |
|
㉟-1. |
204 |
116 (56.9) |
4.2 |
2.37 |
|
㉟-2. |
243 |
167 (68.7) |
3.6 |
2.45 |
|
㉟-3. |
227 |
83 (36.6) |
2.9 |
1.05 |
|
㊱-1. |
322 |
204 (63.4) |
4.6 |
2.95 |
|
㊱-2. |
241 |
156 (64.7) |
3.8 |
2.47 |
|
㊲ |
180 |
180 (100) |
119.1 |
119.07 |
|
㊳ |
74 |
40 (54.1) |
5.8 |
3.12 |
|
|
Streams in east coastal areas |
|
㊴ |
38 |
38 (100) |
42.6 |
42.58 |
|
㊵ |
15 |
13 (86.7) |
13.8 |
11.96 |
|
㊶ |
45 |
36 (80.0) |
4.9 |
3.88 |
|
㊷ |
67 |
56 (83.6) |
9.3 |
7.73 |
|
㊸ |
41 |
24 (58.5) |
2.4 |
1.40 |
|
㊹ |
80 |
42 (52.5) |
3.3 |
1.76 |
Table 6Distributions of susceptibility of Metagonimus spp. metacercariae (MsMcs) in index fish, Zacco spp., by the water systems in Korea
Table 6
|
Locality (River) surveyed |
No. of localities by the susceptibilitya in index fish |
|
Very low |
Low |
Moderate |
High |
Total (%) |
|
Hantan-gang and Imjin-gang |
1 |
1 |
1 |
1 |
4 (8.0) |
|
Han-gang |
0 |
2 |
2 |
1 |
5 (10.0) |
|
Geum-gang |
3 |
1 |
2 |
0 |
6 (12.0) |
|
Mangyeong-gang |
1 |
1 |
0 |
0 |
2 (4.0) |
|
Yeongsan-gang |
0 |
2 |
0 |
1 |
3 (6.0) |
|
Tamjin-gang |
0 |
0 |
3 |
0 |
3 (6.0) |
|
Seomjin-gang |
1 |
5 |
5 |
1 |
12 (24.0) |
|
Nakdong-gang |
7 |
1 |
0 |
1 |
9 (18.0) |
|
Streams in east coast |
3 |
2 |
1 |
0 |
6 (12.0) |
|
Total (%) |
16 (32.0) |
15 (30.0) |
14 (28.0) |
5 (10.0) |
50 (100) |
Table 7Infection status of Metagonimus spp. metacercariae (MsMc) of sweet smelt Plecoglosus altivelis, in the most susceptible fish host, by survey localities
Table 7
|
Locality surveyed |
No. fish examined |
No. (%) fish infected |
No. MsMc detected |
|
|
Range |
Average |
|
Tamjin-gang |
|
㉑-1 (Jangheung) |
40 |
39 (97.5) |
1–5,320 |
1,290 |
|
㉑-2 (Jangheung) |
14 |
14 (100) |
80–5,280 |
1,476 |
|
㉒ (Gangjin) |
40 |
40 (100) |
26–4,280 |
841 |
|
|
Seomjin-gang |
|
㉔-1 (Sunchang) |
3 |
3 (100) |
282–6,750 |
3,146 |
|
㉖-1 (Gokseong) |
2 |
2 (100) |
1,370–4,380 |
2,875 |
|
㉖-2 (Gokseong) |
16 |
5 (31.3) |
1–16 |
7 |
|
㉗ (Gurye) |
6 |
6 (100) |
3,250–16,830 |
8,563 |
|
㉘ (Hwagye-cheon) |
16 |
16 (100) |
1,310–17,750 |
6,830 |
|
㉙ (Akyang-cheon) |
2 |
2 (100) |
3,565–4,865 |
4,215 |
|
|
Nakdong-gang |
|
㊲ (Deokcheon-gang) |
41 |
40 (97.6) |
3–20,650 |
3,570 |
|
|
Water systems in the eastern coastal regions |
|
㊴ (Namdae-cheon) |
15 |
15 (100) |
49–6,280 |
1,522 |
|
㊵ (Osip-cheon) |
3 |
2 (66.7) |
14–21 |
18 |
|
㊶ (Whangpi-cheon) |
59 |
59 (100) |
2–6,199 |
939 |
|
㊷ (Osip-cheon) |
73 |
65 (89.0) |
1–597 |
63 |
|
㊹ (Cheokgwa-cheon) |
16 |
16 (100) |
50–1,920 |
331 |
Table 8Fish species highly infected with Metagonimus spp. metacercariae (MsMc) excluding sweet smelt
Table 8
|
Fish species |
Locality (no. fish positive/no. fish examined mean no. MsMc detected) surveyed |
|
Zacco platypus
|
③ Togyo-jeosuji (137/139; 144), ⑨ Dal-cheon (15/15; 126), ⑪ Chogang-cheon (3/3; 102), ⑳ Yeongam-cheon (15/15; 104), ㊲ Deokcheon-gang (62/62; 106) |
|
Zacco koreanus
|
⑰-2. Soyang-cheon (25/28; 90), ㉙ Akyang-cheon (40/40; 98), ㉚ Namsan-cheon (74/74; 104), ㊲ Hoeng-cheon (70/70; 273), ㊲ Deokcheon-gang (118/118; 126) |
|
Zacco temminckii
|
⑥ Joyang-gang (38/39; 130), ⑳ Yeongam-cheon (4/4; 958), ㉑-2. Tamjin-gang (112/119; 113), ㉒Tamjin-gang (25/26; 110), ㉚ Namsan-cheon (19/19; 97), ㉛ Hoeng-cheon (10/10; 157) |
|
Opsariichthys uncirostris
|
⑧ Seom-gang (1/1; 260), ⑨ Dal-cheon (1/1; 380), ⑬ Geum-gang (8/8; 88), ㉙-1 Seomjin-gang (2/2; 387), ㉗ Seomjin-gang (13/13; 144), ㉞ Yongjeon-cheon (3/3; 89) |
|
Rhynchocypris oxycephalus
|
㉟-3. Wi-cheon (4/4; 747), ㊹Cheokgwa-cheon (20/21; 266) |
|
Carassius auratus
|
㉑-1. Tamjin-gang (51/75; 156) |
|
Acheilognathus rhombeus
|
㉑-1. Tamjin-gang (5/5; 501), ㉗Seomjin-gang (1/1; 185) |
|
Onchorhynchus masou
|
㊴ Namdae-cheon (6/6; 200), ㊵ Osip-cheon (8/8; 253) |
|
Tribolodon hakonensis
|
㊴ Namdae-cheon (2/2; 245), ㊶ Wangpi-cheon (16/16; 115) |
Table 9The fish intermediate hosts of Metagonimus spp. in Korea
Table 9
|
Family |
Genus |
Speciesa
|
|
Cyprinidae |
Abbottina
|
A. rivularis, A. springeri
|
|
Acanthorhodeus
|
A. gracilis, A. macropterusb
|
|
Acheilognathus
|
A. asmussi, A. koreensisb, A. lanceolate, A. majusculusb, A. rhombeus, A. signiferb, A. somjinensisb, A. yamatsutae
|
|
Aphyocypris
|
A. chinensis
|
|
Carassius
|
C. auratus
|
|
Coreoleuciscus
|
C. splendidus
|
|
Cyprinus
|
C. carpio
|
|
Gnathopogon
|
G. strigatus
|
|
Gobiobotia
|
G. brevibarba
|
|
Hemibarbus
|
H. labeo, H. longirostris, H. mylodonb
|
|
Hemiculter
|
H. eigenmanni, H. leucisculusb
|
|
Ladislabia
|
L. taczanowskiib
|
|
Microphysogobio
|
M. koreensisb, M. longidorsalisb, M. yaluensis
|
|
Opsariichthys
|
O. uncirostris amurensis
|
|
Pseudogobio
|
P. esocinus
|
|
Pseudopuntungia
|
P. nigra, P. tenuicorpab
|
|
Pseudorasbora
|
P. parva
|
|
Puntungia
|
P. herzi
|
|
Rhodeus
|
R. ocellatus, R. pseudosericeusb, R. uyekii
|
|
Rhynchocypris
|
R. oxycephalus
|
|
Sarcocheilichthys
|
S. nigripinnis morii, S. variegatus wakiyae
|
|
Squalidus
|
S. japonicus coreanusb, S. gracilis majimae, |
|
S. chankaensis tsuchigae, S. multimaculatusb
|
|
Tribolodon
|
T. hakonensis
|
|
Zacco
|
Z. platypus, Z. temminckii, Z. koreanusb
|
|
|
Amblycipitidae |
Liobagrus
|
L. andersonib, L. mediadiposalis, L. somjinensisb
|
|
Bagridae |
Coreobagrus
|
C. brevicorpus
|
|
Pseudobagrus
|
P. fulvidraco, P. koreanusb
|
|
|
Channidae |
Channa
|
C. argusb
|
|
|
Centrarchidae |
Lepomis
|
L. macrochirus
|
|
Micropterus
|
M. salmoidesb
|
|
|
Cobitidae |
Cobitis
|
C. sinensisb
|
|
Iksookimia
|
I. koreensis
|
|
Koreocobitis
|
K. naktongensisb
|
|
Misgurnus
|
M. anguillicaudatus
|
|
|
Cottidae |
Cottus
|
C. hangiogensisb
|
|
|
Gobiidae |
Acanthogobius
|
A. lactipesb
|
|
Gymnogobius
|
G. urotaeniab
|
|
Rhinogobius
|
R. brunneus
|
|
Tridentiger
|
T. brevispinisb
|
|
|
Lateolabracidae |
Lateolabrax
|
L. japonicus
|
|
|
Mugilidae |
Mugil
|
M. cephalusb
|
|
|
Odontobutidae |
Odontobutis
|
O. interruptab, O. platycephalab
|
|
|
Osphronemidae |
Macropodus
|
M. ocellatus
|
|
|
Plecoglossidae |
Plecoglossus
|
P. altivels
|
|
|
Salmonidae |
Oncorhynchus
|
O. masou masoub
|
|
|
Sinipercidae |
Coreoperca
|
C. herzi
|
|
Siniperca
|
S. scherzei
|
References
- 1. Korea Centers for Disease Control and Prevention. Korea national institute of health. National survey of the prevalence of intestinal parasitic infections in Korea, 2012. The 8th Report. Osong Chungcheongbuk-do; Korea. 2013.
- 2. Seo BS, Lee SH, Cho SY, Chai JY, Hong ST, et al. An epidemiologic study on clonorchiasis and metagonimiasis in riverside areas in Korea. Parasites Hosts Dis 1981;19(2):137-150. https://doi.org/10.3347/kjp.1981.19.2.137
- 3. Cho SH, Lee KY, Lee BC, Cho PY, Cheun HI, et al. Prevalence of clonorchiasis in southern endemic areas of Korea in 2006. Parasties Hosts Dis 2008;46(3):133-137. https://doi.org/10.3347/kjp.2008.46.3.133
- 4. Kim HK, Cheun HI, Chung BS, Lee KY, Kim TS, et al. Prevalence of Clonorchis sinensis infections along the five major rivers in republic of Korea, 2007. Osong Public Health Res Perspect 2010;1(1):43-49. https://doi.org/10.1016/j.phrp.2010.12.010
- 5. June KJ, Cho SH, Lee WJ, Kim C, Park KS. Prevalence and risk factors of clonorchiasis among the populations served by primary healthcare posts along five major rivers in South Korea. Osong Public Health Res Perspect 2013;4(1):21-26. https://doi.org/10.1016/j.phrp.2012.12.002
- 6. Jeong YI, Shin HE, Lee SE, Cheun HI, Ju JW, et al. Prevalence of Clonorchis sinensis infection among residents along 5 major rivers in the Republic of Korea. Parasites Hosts Dis 2016;54(2):215- 219. https://doi.org/10.3347/kjp.2016.54.2.215
- 7. Lee SE, Shin HE, Lee MR, Kim YH, Cho SH, et al. Risk factors of Clonorchis sinensis human infections in endemic areas, Haman-gun, Republic of Korea: a case-control study. Parasites Hosts Dis 2020;58(6):647-652. https://doi.org/10.3347/kjp.2020.58.6.647
- 8. Hong JH, Seo M, Oh CS, Chai JY, Shin DH. Metagonimus yokogawai ancient DNA recovered from 16th- to 17th-century Korean mummy feces of the Joseon Dynasty. J Parasitol 2020;106(6):802-808. https://doi.org/10.1645/20-42
- 9. Oh CS, Hong JH, Chai JY, Song MK, Jang HJ, et al. Ancient DNA of Metagonimus yokogawai recovered from Joseon period human remains newly discovered at Goryeong County in South Korea. Acta Parasitol 2022;67(1):539-545. https://doi.org/10.1007/s11686-021-00487-0
- 10. Chai JY. Metagonimus. In Xiao L, Ryan U, Feng Y eds, Biology of Foodborne Parasites. CRC Press; Boca Raton, USA. 2015, pp 427-443 https://doi.org/10.1201/b18317
- 11. Chai JY, Jung BK. Fishborne zoonotic heterophyid infections: an update. Food Waterborne Parasitol 2017;8(9):33-63. https://doi.org/10.1016/j.fawpar.2017.09.001
- 12. Shumenko PG, Tatonova YV, Besprozvannykh VV. Metagonimus suifunensis sp. n. (Trematoda: Heterophyidae) from the Russian Southern Far East: morphology, life cycle, and molecular data. Parasitol Int 2017;66(1):982-991. https://doi.org/10.1016/j.parint.2016.11.002
- 13. Tatonova YV, Shumenko PG, Besprozvannykh VV. Description of Metagonimus pusillus sp. nov. (Trematoda: Heterophyidae): phylogenetic relationships within the genus. J Helminthol 2018;92(6):703-712. https://doi.org/10.1017/S0022149X17001146
- 14. Chai JY, Cho SY, Seo BS. Study on Metagonimus yokogawai (Katsurada, 1912) in Korea. IV. An epidemiological investigation along Tamjin river basin, South Cholla Do, Korea. Parasites Hosts Dis 1977;15(2):115-120. https://doi.org/10.3347/kjp.1977.15.2.115
- 15. Soh CT, Ahn YK. Epidemiological study on Metagonimus yokogawai infection along Boseong River in Jeonra Nam Do, Korea. Parasites Hosts Dis 1978;16(1):1-13. (in Korean). https://doi.org/10.3347/kjp.1978.16.1.1
- 16. Kim DC, Lee OY, Jeong EB, Han EJ. Epidemiological conditions of Metagonimus yokogawai infection in hadong gun, Gyeongsang Nam Do. Parasites Hosts Dis 1979;17(1):51-59. (in Korean). https://doi.org/10.3347/kjp.1979.17.1.51
- 17. Ahn YK. Epidemiological studies on Metagonimus yokogawai infection in Samcheok-Gun, Kangwon-do, Korea. Parasites Hosts Dis 1984;22(2):161-170. (in Korean). https://doi.org/10.3347/kjp.1984.22.2.161
- 18. Ahn YK, Chung PR, Lee KT, Soh CT. Epidemiological survey on Metagonimus yokogawai infection in the eastern coast area of Kangwon Province, Korea. Parasites Hosts Dis 1987;25(1):59-68. (in Korean). https://doi.org/10.3347/kjp.1987.25.1.59
- 19. Chai JY, Han ET, Park YK, Guk SM, Kim JL, et al. High endemicity of Metagonimus yokogawai infection among residents of Samchok-shi, Kangwon-do. Parasites Hosts Dis 2000;38(1):33-36. https://doi.org/10.3347/kjp.2000.38.1.33
- 20. Lee JJ, Kim HJ, Kim MJ, Lee JWY, Jung BK, et al. Decrease of Metagonimus yokogawai endemicity along the tamjin river basin. Parasites Hosts Dis 2008;46(4):289-291. https://doi.org/10.3347/kjp.2008.46.4.289
- 21. Chai JY, Huh S, Yu JR, Kook JN, Jung KC. An epidemiological study of metagonimiasis along the upper reaches of the Namhan River. Parasites Hosts Dis 1993;31(2):99-108. https://doi.org/10.3347/kjp.1993.31.2.99
- 22. Kim CH. Study on the Metagonimus sp. in Gum river basin, Chungchungnam-do, Korea. Parasites Hosts Dis 1980;18(2):215-228. (in Korean). https://doi.org/10.3347/kjp.1980.18.2.215
- 23. Kim CH, Kim NM, Lee CH, Park JS. Study on the Metagonimus fluke in the daecheong reservoir and the upper stream of Gum river, Korea. Parasites Hosts Dis 1987;25(1):69-82. (in Korean). https://doi.org/10.3347/kjp.1987.25.1.69
- 24. Lee GS, Cho IS, Lee YH, Noh HJ, Shin DW, et al. Epidemiological study of clonorchiasis and metagonimiasis along the Geum-gang (River) in Okcheon-gun (County), Korea. Parasites Hosts Dis 2002;40(1):9-16. https://doi.org/10.3347/kjp.2002.40.1.9
- 25. Katsurada F. On the pathogenic human trematodes in the Far East. J Chosen Med Ass 1913;6.
- 26. Sohn WM. Fish-borne zoonotic trematode metacercariae in the Republic of Korea. Parasites Hosts Dis 2009;47(suppl):S103-S113. https://doi.org/10.3347/kjp.2009.47.S.S103
- 27. Chun SK. A study on Metagonimus yokogawai from plecoglossus altivelis in the miryang river. Bull Pusan Fish Coll 1960a;3:24-30. (in Korean).
- 28. Chun SK. A study on the metacercaria of Metagonimus takahashii and Exorchis oviformis from Carassius carassius. Bull Pusan Fish Coll 1960b;3:31-39. (in Korean).
- 29. Kang SY, Loh IK, Park BC, Rim DB. Studies on Metagonimus yokogawai infected in Plecoglossus altivelis collected in Che-ju Province. J Korean Med Assoc 1964;7:470-476. (in Korean).
- 30. Choi DW, Lee JT, Hwang HK, Shin YD. Studies on the larval trematodes from brackish water fishes 2. Observation on Metagonimus yokogawai Katsurada, 1912. Parasites Hosts Dis 1966;4(1):33-37. (in Korean). https://doi.org/10.3347/kjp.1966.4.1.33
- 31. Chun SK. Studies on some trematodes whose intermediate hosts are fishes in the Naktong River. Bull Pusan Fish Coll 1962;4:21-38.
- 32. Lee JT. Studies on the metacercariae from freshwater fishes in the Kumho River. Parasites Hosts Dis 1968;6(3):77-99. https://doi.org/10.3347/kjp.1968.6.3.77
- 33. Hong NT, Seo BS. Study on Metagonimus yokogawai (Katsurada, 1912) in Korea. I. On the metacercaria, its distribution in the second intermediate host and the development in the final host. Parasites Hosts Dis 1969;7(3):129-142. https://doi.org/10.3347/kjp.1969.7.3.129
- 34. Hwang JT, Choi DW. Metacercarial density of Metagonimus yokogawai in Plecoglossus altivelis in Kyungpook Province, Korea. Parasites Hosts Dis 1977;15(1):30-35. https://doi.org/10.3347/kjp.1977.15.1.30
- 35. Suh JW, Choi DW. Demonstration of Metagonimus yokogawai metacercariae from Plecoglossus altivelis in River Ahnseong, Kyungpook Province, Korea. Parasites Hosts Dis 1979;17(1):45-50. https://doi.org/10.3347/kjp.1979.17.1.45
- 36. Hwang JT, Choi DW. Changing pattern of infestation with larval trematodes from freshwater fish in river Kumho, Kyungpook Province, Korea. Kyungpook Uni Med J 1980;21:460-475.
- 37. Song CY. Studies on the yokogawa’s fluke Metagonimus yokogawai (Katsurada, 1912) in Korea. I. Geographical distribution of sweetfish and their infection status with Metagonimus metacercariae in Gang-Woen Do. Chung-Ang J Med 1981;6:121-126. (in Korean).
- 38. Seo BS, Hong ST, Chai JY, Lee SH. Studies on Metagonimus yokogawai (Katsurada, 1912) in Korea. VI. The geographical distribution of metacercarial infection in sweetfish along the East and South coast. Parasites Hosts Dis 1982;20(1):28-32. (in Korean). https://doi.org/10.3347/kjp.1982.20.1.28
- 39. Rhee JK, Lee HI, Baek BK, Kim PG. Survey on encysted cercariae of trematodes from freshwater fishes in Mangyeong riverside area. Parasites Hosts Dis 1983;21(2):187-192. (in Korean). https://doi.org/10.3347/kjp.1983.21.2.187
- 40. Rhee JK, Rim MH, Baek BK, Lee HI. Survey on encysted cercariae of trematodes from freshwater fishes in Tongjin riverside areas in Korea. Parasites Hosts Dis 1984;22(2):190-202. https://doi.org/10.3347/kjp.1984.22.2.190
- 41. Song CY, Lee SH, Jeon SR. Studies on the intestinal fluke, Metagonimus yokogawai Katsurada, 1912 in Korea. IV. Geographical distribution of sweetfish and infection status with Metagonimus metacercariae in south-eastern area of Korea. Parasites Hosts Dis 1985;23(1):123-138. (in Korean). https://doi.org/10.3347/kjp.1985.23.1.123
- 42. Ahn YK, Ryang YS. Epidemiological studies on Metagonimus infection along the Hongcheon River, Kangwon Province. Parasites Hosts Dis 1988;26(3):207-213. (in Korean). https://doi.org/10.3347/kjp.1988.26.3.207
- 43. Sohn WM, Hong ST, Chai JY, Lee SH. Infection status of sweetfish from Kwangjung-stream and Namdae-stream in Yangyang-gun, Kangwon-do with the metacercariae of Metagonimus yokogawai. Parasites Hosts Dis 1990;28(4):253-255. (in Korean). https://doi.org/10.3347/kjp.1990.28.4.253
- 44. Ahn YK. Intestinal fluke of genus Metagonimus and their second intermediate hosts in Kangwon-do. Parasites Hosts Dis 1993;31(4):331-340. (in Korean). https://doi.org/10.3347/kjp.1993.31.4.331
- 45. Park MS, Kim SW, Yang YS, Park CH, Lee WT, et al. Intestinal parasite infections in the inhabitants along the Hantan River, Chorwon-gun. Parasites Hosts Dis 1993;31(4):375-378. https://doi.org/10.3347/kjp.1993.31.4.375
- 46. Yu JR, Kwon SO, Yu JR, Lee SH. Clonorchiasis and metagonimiasis in the inhabitants along talchongang (River), Chungwon-gun. Parasites Hosts Dis 1994;32(4):267-269. https://doi.org/10.3347/kjp.1994.32.4.267
- 47. Rim HJ, Kim KH, Joo KH. Classification and host specificity of Metagonimus spp. from Korean freshwater fish. Parasites Hosts Dis 1996;34(1):7-14. https://doi.org/10.3347/kjp.1996.34.1.7
- 48. Cho SH, Kim TS, Na BK, Sohn WM. Prevalence of Metagonimus metacercariae in sweetfish, plecoglossus altivelis, from eastern and southern coastal areas in Korea. Parasites Hosts Dis 2011;49(2):161-165. https://doi.org/10.3347/kjp.2011.49.2.161
- 49. Cho SH, Lee WJ, Kim TS, Seok WS, Lee TJ, et al. Prevalence of zoonotic trematode metacercariae in freshwater fish from Gangwon-do, Korea. Parasites Hosts Dis 2014;52(4):399-412. https://doi.org/10.3347/kjp.2014.52.4.399
- 50. Sohn WM, Na BK, Cho SH, Lee SW, Choi SB, et al. Trematode metacercariae in freshwater fish from water systems of Hantan-gang and Imjin-gang in Republic of Korea. Parasites Hosts Dis 2015;53(3):289-298. https://doi.org/10.3347/kjp.2015.53.3.289
- 51. Sohn WM, Na BK, Cho SH, Ju JW, Kim CH, et al. Infection status with Metagonimus spp. metacercariae in fishes from Seomjin-gang and Tamjin-gang in Republic of Korea. Parasites Hosts Dis 2018;56(4):351-358. https://doi.org/10.3347/kjp.2018.56.4.351
- 52. Chai JY, Park JH, Han ET, Shin EH, Kim JL, et al. Prevalence of Heterophyes nocens and Pygidiopsis summa infections among residents of the western and southern coastal islands of the Republic of Korea. Am J Trop Med Hyg 2004;71:617-622.
- 53. Guk SM, Shin EH, Kim JL, Sohn WM, Hong KS, et al. A survey of Heterophyes nocens and Pygidiopsis summa metacercariae in mullets and gobies along the coastal areas of the Republic of Korea. Parasites Hosts Dis 2007;45(3):205-211. https://doi.org/10.3347/kjp.2007.45.3.205
- 54. Park JH, Kim JL, Shin EH, Guk SM, Park YK, et al. A new endemic focus of Heterophyes nocens and other heterophyid infections in a coastal area of Gangjin-gun, Jeollanam-do. Parasites Hosts Dis 2007;45(1):33-38. https://doi.org/10.3347/kjp.2007.45.1.33
- 55. Cho SH, Cho PY, Lee DM, Kim TS, Kim IS, et al. Epidemiological survey on the infection of intestinal flukes in residents of Muan-gun, Jeollanam-do, the Republic of Korea. Parasites Hosts Dis 2010;48(2):133-138. https://doi.org/10.3347/kjp.2010.48.2.133
- 56. Chai JY, Jung BK, Kim DG, Kim JL, Lim H, et al. Heterophyid trematodes recovered from people residing along the boseong river, South Korea. Acta Trop 2015;148:142-146. https://doi.org/10.1016/j.actatropica.2015.04.025
- 57. Sohn WM, Na BK, Cho SH, Kim CH, Hwang MA, et al. Survey of zoonotic trematode metacercariae in fish from water systems of Geum-gang (River) in Republic of Korea. Parasites Hosts Dis 2021;59(1):23-33. https://doi.org/10.3347/kjp.2021.59.1.23
- 58. Sohn WM, Na BK, Cho SH, Lee SW. Infection status with digenetic trematode metacercariae in fishes from coastal lakes in Gangwon-do, Republic of Korea. Parasites Hosts Dis 2019;57(6):681-690. https://doi.org/10.3347/kjp.2019.57.6.681
- 59. Sohn WM, Na BK. Infections with digenetic trematode metacercariae in freshwater fishes from two visiting sites of migratory birds in Gyeongsangnam-do, Republic of Korea. Parasites Hosts Dis 2019;57(3):273-281. https://doi.org/10.3347/kjp.2019.57.3.273
- 60. Sohn WM, Na BK, Cho SH, Ju JW, Kim CH, et al. Prevalence and infection intensity of zoonotic trematode metacercariae in fish from Soyang-cheon (Stream) in Wanju-gun, Jeollabuk-do, Korea. Parasites Host Dis 2021;59(3):265-271. https://doi.org/10.3347/kjp.2021.59.3.265
- 61. Sohn WM, Na BK, Cho SH, Lee HI, Ju JW, et al. Survey of zoonotic trematode metacercariae in fish from irrigation canal of Togyo-jeosuji (Reservoir) in Cheorwon-gun, Gangwon-do, Republic of Korea. Parasites Hosts Dis 2021;59(4):427-432. https://doi.org/10.3347/kjp.2021.59.4.42
- 62. Sohn WM, Na BK, Cho SH, Lee HI, Ju JW, et al. Endemicity of zoonotic trematode metacercariae in fish from Deokcheon-gang (River) in Sancheong-gun, Gyeongsangnam-do, Republic of Korea. Parasites Hosts Dis 2021;59(5):523-529. https://doi.org/10.3347/kjp.2021.59.5.523
- 63. Seo BS, Lee HS, Chai JY, Lee SH. Intensity of Metagonimus yokogawai infection among inhabitants in Tamjin River basin with reference to its egg laying capacity in human host. Seoul J Med 1985;26(2):207-212.
- 64. Yeo TO, Seo BS. Study on Metagonimus yokogawai in Korea III. Epidemiological observation of human infection in Hadong area, South Kyongsang Do. Seoul J Med 1971;12(4):259-267. (in Korean).
- 65. Chai JY, Sohn WM, Kim MH, Hong ST, Lee SH. Three morphological types of the genus Metagonimus encysted in the dace, Tribolodon taczanowskii, caught from the Sumjin River. Parasites Host Dis 1991;29(3):217-225. https://doi.org/10.3347/kjp.1991.29.3.217
- 66. Sohn WM. Infection characteristics of Clonorchis sinensis metacercariae in fish from Republic of Korea. Parasites Hosts Dis 2022;60(2):79-96. https://doi.org/10.3347/kjp.2022.60.2.79
- 67. Joo CY, Park SG. Epidemiological survey of Metagonimus yokogawai in Ulju county, Kyungnam Province, Korea. Kyungpook Univ Med J 1982;23:1-9. (in Korean).
- 68. Joo CY, Park MK, Choi DW. Infestation of larval trematodes from freshwater fish and brackish water fish in River Taechong, Kyungpook Province, Korea. Parasites Hosts Dis 1983;21(1):6-10. (in Korean). https://doi.org/10.3347/kjp.1983.21.1.6
- 69. Joo CY. Infestation of larval trematodes from freshwater fish and brackish water fish in River Hyungsan, Kyungpook Province, Korea. Parasites Hosts Dis 1984;22(1):78-84. (in Korean). https://doi.org/10.3347/kjp.1984.22.1.78
- 70. Joo CY. Changing patterns of infection with digenetic larval trematodes from freshwater fish in River Taewha, Kyungnam Province. Parasites Hosts Dis 1988;26(4):263-274. https://doi.org/10.3347/kjp.1988.26.4.263
- 71. Kim JH, Choi DW. Infestation with larval trematodes from freshwater fish in natural and fish breeding ponds. Parasites Hosts Dis 1981;19(2):157-166. https://doi.org/10.3347/kjp.1981.19.2.157
- 72. Ahn YK. Lateolabrax japonicus, a role of second intermediate host of Metagonimus yokogawai. New Med J 1983;26:135-139.
- 73. Saito S, Chai JY, Kim KH, Lee SH, Rim HJ. Metagonimus miyatai sp. nov. (Digenea: Heterophyidae), a new intestinal trematode transmitted by freshwater fishes in Japan and Korea. Parasites Hosts Dis 1997;35(4):223-232. https://doi.org/10.3347/kjp.1997.35.4.223
- 74. Kim DG, Kim TS, Cho SH, Song HJ, Sohn WM. Heterophyid metacercarial infections in brackish water fishes from Jinju-man (Bay), Kyongsangnam-do, Korea. Parasites Hosts Dis 2006;44(1):7-13. https://doi.org/10.3347/kjp.2006.44.1.7
- 75. Saito S, Shimizu T. A new trematode, Metagonimus otsurui sp. nov. from the freshwater fishes (Trematoda: Heterophyidae). Jpn J Parasitol 1968;17:167-174.
- 76. Oyamada T, Kudo N, Kitahara T, Takatou Y. Metagonimus otsurui metacercarial infection in gobiid fish (Tridentiger brevispinis) collected from Lake Ogawara in Aomori Prefecture, Japan. Jpn J Parasitol 1996;45(4):275-279.
- 77. Shimazu T, Urabe M. Morphology and life cycle of Metagonimus otsurui (Digenea: Heterophyidae) from Naga, Japan. Bull Natn Sci Mus Tokyo 2002;28(1):21-28.